An Unexpected Pairing of Frustrated Molecules
Scientists at PNNL explain how separated molecules get together to split hydrogen
(January 2013)

The research graced the cover of the Dalton Transactions themed issue on boranes and borohydrides. Permission to use granted by the Royal Society of Chemistry.
Results: While their shapes frustrate traditional bonding, two unreactive molecules come together and surround themselves within a solvent cage to create a reactive environment to split hydrogen, according to two new studies by scientists at Pacific Northwest National Laboratory. Splitting a hydrogen molecule into a proton and a hydride ion (H-), known as activating the hydrogen, is vital for sustainable energy production and storage. The pair of molecules is called a frustrated Lewis pair or FLP.
"The conventional wisdom says that frustrated Lewis pairs should not be able to activate hydrogen-but they do. We wanted to know why," said Dr. Greg Schenter, a theoretical chemist on this project.
Why It Matters: Turning plant material or other renewable resources into fuels requires adding hydrogen with minimal use of external energy. This demands an effective catalyst. The studies provide fundamental insights into the processes that could one day be used to optimize that catalyst.
"This is very fundamental research," said Dr. Shawn Kathmann, a PNNL theoretician on the project. "It is advancing our struggle to understand more complex catalyzed reactions."
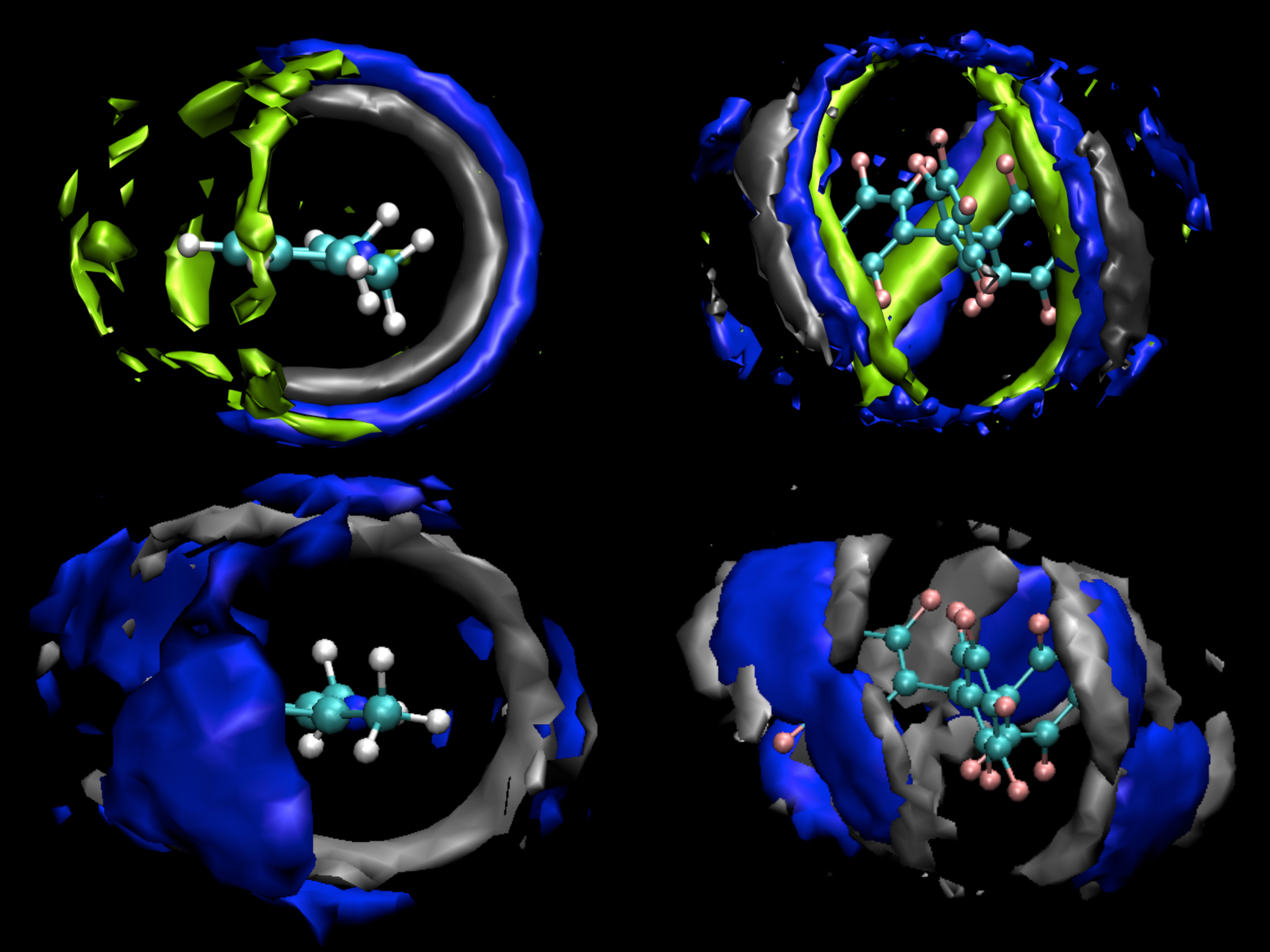
At Pacific Northwest National Laboratory, researchers built simulations showing how two molecules combine to activate hydrogen, shedding new light on a reaction that could, one day, support hydrogenation of biofuels.
Methods: The theoreticians were motived by their conversations with Dr. Thomas Autrey, an experimental chemist who was working down the hall on characterizing and measuring hydrogen activation by FLP. "What we and others are seeing in the lab cannot be readily explained," said Autrey.
The team began modeling the systems Autrey had been studying. "Modeling allows us to see what experiments can't," said Schenter.
The team created three models: the two molecules that make up the FLP, the liquid environment they reside in, and finally the FLP in the liquid. The molecules that made up the FLP were 2,6-lutidine and tris(pentafluorophenylborane). The team compared two solvents, toluene and dichlormethane, for the liquid environment or solvent. These solvents initially hinder the pair getting together, but also enable the pair to have a finite lifetime within the solvent cage, sufficiently long to allow hydrogen to diffuse into the cage to be activated by the FLP. The balance of these competing reactions is directly related to the tumbling rates of all the components in the solvent. The team calculated the tumbling rate of these molecules and studied how they reorganized, or didn't, during the reaction.
"We constructed a reasonable description of the interactions, put it all together, and then watched what happened," said Dr. Liem Dang, an expert computational modeler of molecular interactions and the project lead.
At first, the watching took a long time. So, the team biased the model to get the pair of molecules to come together quickly and then calculated how long it would take the pair to come together without bias. "The modeling gives you a lot of information about how fast the molecules will come together," said Schenter.
The solvent forms a cage or void around the molecules. The solvent drags on the molecules, but they pair up to form a more stable solvent structure. Occasionally, the team saw a single molecule of solvent trapped between a pair coming together. However, the pair overcame the obstacle and ejected the lone solvent molecule. Once together, the pair provides a unique environment to split hydrogen molecules into a hydride and proton pair.
What's Next? The team is continuing to conduct theoretical and experimental studies of nonmetal catalysts. Currently, they are measuring the rotational motion of solvent molecules, solute molecules, and combinations of solutes and solvents using nuclear magnetic resonance spectroscopy. The goal of their work is to explain how this unexpected reaction works to develop rational approaches to optimize catalytic reaction pathways.
Acknowledgments:
Sponsor: Both studies were funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. On the study published in Dalton Transactions, the Laboratory Directed Research and Development Program at PNNL provided funding for the kinetic analyses of the calorimetry data.
Research Area: Chemical Sciences
User Facility: Environmental Molecular Sciences Laboratory
Research Team: Liem X. Dang, Gregory K. Schenter, Tsun-Mei Chang, Shawn M. Kathmann, Tom Autrey, Abhi Karkamkar, Kshitji Parab, Donald Camaioni, Doinita Neiner, and Herman Cho, PNNL; Thomas Nielsen, Aarhus University in Denmark. Tsun-Mei Chang was a summer visitor from University of Wisconsin and was funded by the catalysis effort to help develop the capabilities that were used in this study.
References: Dang LX, GK Schenter, TM Chang, SM Kathmann, and T Autrey. 2012. "Role of Solvents on the Thermodynamics and Kinetics of Forming Frustrated Lewis Pairs." Journal of Physical Chemistry Letters 3(22):3312-3319. DOI: 10.1021/jz301533a
Karkamkar A, K Parab, DM Camaioni, D Neiner, H Cho, TK Nielsen and T Autrey. 2013. "A Thermodynamic and Kinetic Study of the Heterolytic Activation of Hydrogen by Frustrated Borane-Amine Lewis Pairs." Dalton Transactions 42:615-619. DOI: 10.1039/c2dt31628e
