Strong Influence of a Minor Substitution in Gold Clusters
Ligand change controls size, stability, and charge state of nanoparticles synthesized in solution
(October 2013)
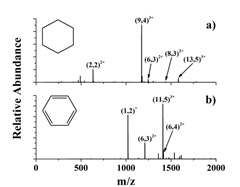
Using a combination of synthesis in solution and high-resolution mass spectrometry, scientists at PNNL demonstrated that the substitution of diphosphine ligands (top: phenyl; bottom: cyclohexane) dramatically influences the size and fragmentation pathways of otherwise identical gold clusters. Enlarge Image | Flier | Slide
Results: What could you change about our nation's energy, transportation, and other sectors if you could synthesize a material with exactly the properties that you needed on demand and with high purity? The answer to creating these materials may lie in sub-nanometer building blocks called clusters that have a precisely defined size and composition. Producing commercially relevant quantities involves the reduction in solution of positively charged metal ions to neutral atoms in the presence of organic molecules called ligands, which arrest the growth at a particular number of metal atoms. Researchers at Pacific Northwest National Laboratory showed that changes at a diphenylphosphine ligand's phosphorus centers alter the size of clusters synthesized in solution and the fragmentation pathways of otherwise identical clusters.
"Until now, scientists assumed that substitutions in certain ligands did not influence the size or stability of clusters formed," said Dr. Julia Laskin, a PNNL physical chemist involved with the project.

To reduce industry’s energy demands, scientists must build innovative materials; this research provides foundational insights into directing atomic clusters to create these materials.
Why It Matters: Future materials that will decrease energy consumption and waste associated with manufacturing, detect harmful contaminants in foods and medicines, and enable the ultimate miniaturization of complex electronic devices will be composed of clusters containing different numbers and types of atoms. Clusters exhibit large variations in critical properties, such as electron affinity and ionization energy with changing size that may be exploited to tune their stability and chemical reactivity for specific applications.
"By understanding the fundamentals of cluster synthesis, we may control and exploit them to create these superior materials," said Dr. Grant Johnson, a physical chemist at PNNL who led the study.
Methods: Diphenylphosphine ligands, which consist of two phenyl (C6H5) substituted phosphorus centers separated by a carbon chain of variable length, produce gold clusters with extremely narrow distributions in size; that is, the synthesis route produces a large quantity of clusters with the same number of gold atoms as well as a small number of clusters with similar numbers of atoms. The length of the carbon chain between the two phosphorus centers determines the size and monodispersity of the gold clusters. In contrast, the substituents (e.g., phenyl (C6H5)) at the two phosphorus centers are generally regarded as not exerting an influence on the selectivity of diphosphine ligands towards gold clusters of a particular size. Furthermore, theoretical calculations on diphosphine capped gold clusters often involve the substitution of simpler hydrogen atoms in the place of computationally demanding substituents such as phenyl groups with the assumption that the substitution does not alter the stability of the cluster.
The team wanted to see if this assumption would hold up. They focused on gold clusters capped with diphosphine ligands. The team prepared two gold cluster solutions that differed only in the substitution of either cyclohexane groups or phenyl groups at the phosphorus centers of diphosphine ligands.
Thomas Priest, a DOE Science Undergraduate Laboratory intern at PNNL, synthesized the gold clusters, creating a purple solution that Johnson electrosprayed into a high-resolution mass spectrometer at EMSL. The electrospray process turns the gold clusters in the liquid into positively charged ions in a gaseous stream. The team analyzed the composition of the solution including the number of gold atoms and ligands in each cluster using the mass spectrometer. In addition, they isolated representative ions with the instrument and fragmented these clusters through high kinetic energy collisions with an inert background gas to provide insight into the structure and stability of the clusters.
They found that clusters synthesized using the phenyl ligand contained predominantly 11 gold atoms. In contrast, clusters prepared using the cyclohexane ligand contained 9 gold atoms. In addition, gold clusters with the phenyl-containing ligand fragmented through a wide range of dissociation channels involving the loss of gold atoms as well as activation of the phosphorus-carbon bonds of the ligands. The gold clusters with the cyclohexane ligands exhibited far less activation of the ligands. By swapping cyclohexane (C6H11) for phenyl (C6H5) groups in otherwise identical diphosphine ligands, the team showed that gold clusters of completely different size may be synthesized in solution under the same reaction conditions.
"This work demonstrates that the substitution of the phosphorus centers is another powerful parameter that may be used to direct the size-selected synthesis of a broad range of gold clusters." said Johnson.
What's Next? Johnson and members of Laskin's team are now studying how the composition of the solutions evolves from the initial stages of reaction through the formation of the final products. These kinetic studies are aimed at determining the appropriate conditions of reaction time, temperature and concentration to produce a continuous flow of a wide array of different size clusters for studies in the gas-phase using mass spectrometry and on surfaces through soft landing of mass selected ions.
Acknowledgments:
Funding: DOE Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Linus Pauling Fellowship Program at PNNL, Laboratory Directed Research and Development Program at PNNL and DOE Science Undergraduate Laboratory Internship Program at PNNL.
Research Team: Grant Johnson, Thomas Priest, and Julia Laskin, PNNL.
User Facility: EMSL, Environmental Molecular Sciences Laboratory.
Reference: Johnson GE, T Priest, and J Laskin. 2013. "Synthesis and Characterization of Gold Clusters Ligated with 1,3-Bis(dicyclohexylphosphino)propane." ChemPlusChem 78:1033-1039. DOI: 10.1002/cplu.201300134
Related Highlights: Soft Landing and Particle Coverage Key to Keeping or Losing Charge on Surfaces
