Krypton Reporter Uncovers Oxygen's Antics
On the surface of a popular catalyst, certain atoms and molecules flee when light appears
(March 2014)
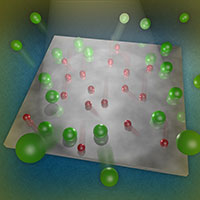
When light strikes oxygen adatoms (red) on the surface of a titanium dioxide catalyst, the adatoms are excited by reactions with electrons and/or holes created in the catalyst. The adatoms undergo a change in their charge state and transfer energy to nearby krypton atoms (green), causing the reporting atoms to depart. A similar result was seen for chemisorbed molecular oxygen. Enlarge image
Results: Long thought to be unresponsive to ultraviolet light, negatively charged oxygen ions stuck to the catalyst's surface, known as oxygen adatoms, actually respond to light, according to scientists at Pacific Northwest National Laboratory (PNNL). The researchers made this discovery by coating the surface of common catalyst titanium dioxide with krypton reporters. When light strikes the catalyst, the oxygen adatoms become electronically excited and knock the krypton off the surface. The alteration occurs because the adatoms react with electrons and/or holes created by the ultraviolet light.
"The adatoms could be an additional source of photochemical interactions on titanium dioxide or other transition metal surfaces," said Dr. Nikolay Petrik, a physical chemist at PNNL and one of two authors on the yearlong study. "Potentially, the adatoms could participate in other photochemical reactions."
Why It Matters: Despite being part of numerous devices and industrial processes, photocatalytic reactions on metal oxides, such as titanium dioxide, are not well understood. This study answers basic questions about the behavior of oxygen adatoms and other forms of chemisorbed oxygen, where the oxygen is adsorbed onto the surface and held by chemical bonds. Fundamental knowledge could lead to innovations in catalysis and energy production technologies, such as solar and fuel cells. "Oxygen on titanium dioxide is important because most applications involve oxygen in some way or another," said Dr. Greg Kimmel, a PNNL chemical physicist and the other scientist on the study.
Methods: A challenge in studying the photochemistry of titanium dioxide, such as the light-induced breakdown of water or organic solvents, is that the oxygen adatoms and other forms of chemisorbed oxygen stay put. Most of the chemisorbed oxygens do not desorb thermally, and it is difficult to analyze the oxygen using other techniques. "There are few tools available to tell scientists what is happening with oxygen on the surface," said Petrik. "What we were looking for was a way to probe oxygen left on the surface."
To examine the oxygen's behavior, Petrik came up with the idea of adding reporter molecules to the surface and seeing what they would tell. Reporter molecules have been used for decades, but they had not been applied to this situation. Working together, Petrik and Kimmel began with a slightly reduced crystal of titanium dioxide. They absorbed different forms of chemisorbed oxygen, including oxygen adatoms and molecular oxygen, onto the crystals. Next, they added weakly bonding krypton and shined light on the surface. They found the krypton is knocked off the surface and can be easily measured.
Petrik offers this analogy for understanding the technique. Imagine a large room with an invisible person in it. How do you determine their location, their choices? You could fill the room with balloons. While you can't see the person move, you can determine their actions by the movement of the "reporter" balloons. Photo-active molecules, such as oxygen adatoms or molecular oxygen (O2), are like that invisible person. The departure of the krypton tells the scientists about how the oxygen prowls across the surface.
In this case, the oxygen adatoms are excited by reactions with electrons and/or holes created in the substrate by ultraviolet photon irradiation. The oxygen adatoms undergo a change in their charge state. This change causes the adatoms to move and collide with, and transfer energy to, nearby krypton atoms, overcoming the atoms' binding energy and desorbing them from the surface. The krypton reporters did not depart under other circumstances, including when ultraviolet light hit the surface without adsorbed oxygen. Further, the krypton's departure was not primarily due to its collision with the 11 to 50 percent of molecular oxygen that photo-desorbs.
What's Next? In regard to oxygen's behavior, Petrik and Kimmel are studying reactions of oxygen with considerably more complicated molecules, such as acetone, via infrared spectroscopy.
Acknowledgments:
Sponsor: U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences & Biosciences Division
User Facility: EMSL
Research Area: Chemical Sciences
Research Team: Nikolay G. Petrik and Greg A. Kimmel, PNNL
Reference: Petrik NG and GA Kimmel. 2014. "Probing the Photochemistry of Chemisorbed Oxygen on TiO2(110) with Kr and Other Co-Adsorbates." Physical Chemistry Chemical Physics 16:2338-2346. DOI: 10.1039/c3cp54195a
