Getting into the Fast Lane Means Minor Adjustments Far from the Catalyst's Core
Adding an amino acid increases speed by 400% and requires little energy
(May 2014)
Including an arginine in the ligand of a hydrogen oxidation catalyst results in water solubility, enhances proton movement through the carboxylic acid groups, and alters that active site structure through intramolecular guanidinium:guanidinium interactions. Enlarge Image.
Results: When it comes to making catalysts that quickly snap chemical bonds and free the stored energy, researchers often focus on the active site. However, small changes far from the active site can also have a large impact. Taking a cue from enzymes, researchers at Pacific Northwest National Laboratory (PNNL) placed the amino acid arginine at the periphery of a hydrogen-splitting catalyst that cleaves hydrogen into protons and electrons. The arginine's carboxylic acid groups accelerated proton transfer and made the catalyst more energy efficient. At the same time, the arginine guanidinium groups interacted with each other to increase the rate of hydrogen binding and activation. With the appended arginine and at 133 atm hydrogen, this nature-inspired catalyst can split 144,000 molecules of hydrogen in a single second.
"Here we are incorporating minimal elements of natural enzymes into synthetic catalysts to understand their role and begin defining how much is necessary to make molecular catalysts as good as, or better than, the enzyme," said Dr. Arnab Dutta, a postdoctoral fellow at PNNL who worked on the study.
Why It Matters: Few people want electricity only when the sun shines. Solar and wind energy could play a larger role if the energy they capture were to be stored in the chemical bonds of fuels such as hydrogen in fuel cells, then recovered later as electricity. Hydrogenase enzymes do exactly this, and quickly: some can cleave 10,000 molecules of hydrogen a second with little excess energy and without precious metals such as platinum. The enzyme is made up of an active site and a protein scaffold. The active site is where the action is; however, the core or active site is not the whole story.
The PNNL team hypothesized that to mimic enzymes' speed and energy efficiency, they must keep selective parts of the protein scaffold or outer coordination sphere, in this case a single amino acid. With this study, scientists are getting closer to enzymatic speed and energy efficiency -- all with changes far from the active site, and in a system that is much more stable than the enzyme under fuel cell conditions.
Methods: The team began with the nickel-based DuBois catalyst, which has a proton relay. They added a second proton relay to build a proton channel that moves protons from the active site to the surrounding solution. Within these channels, the proton is passed from one relay to the next like a baton in a relay race. The team added arginine to the complex, with the idea of putting another player in the race.
The revised catalyst is abbreviated [NiII(PCy2NArg2)2]8+; Arg stands for arginine.
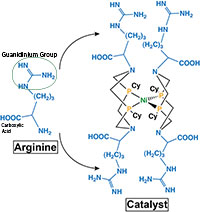
Structure of arginine, with guanidinium group and carboxylic acid noted, and the catalyst. Enlarge Image.
Arginine was chosen because it plays important roles in moving protons and stabilizing structures in proteins and enzymes. In the synthetic catalyst, this amino acid's carboxylic acid groups make protons move quickly, decreasing the excess energy required for catalysis. The [NiII(PCy2NArg2)2]8+ system is one of the most energy-efficient synthetic catalysts now known.
"Energy efficiency is extremely relevant to energy technologies, and our research is allowing us to put together a general understanding of what affects or alters energy efficiency," said Dr. John A. S. Roberts, a PNNL chemist who worked on the study.
While the arginines' guanidinium groups were introduced with the idea of further enhancing proton movement, they serve a different role, interacting to cause a change in the shape of the active site, resulting in a catalyst that splits 210 hydrogen molecules a second at 1 atm hydrogen, four times faster than previously reported complexes. When the hydrogen pressure was increased to 133 atm, the catalyst breaks 144,000 molecules a second, orders of magnitude faster.
In some ways, the team's catalyst is better suited for real-world energy technologies than its natural inspiration, because extreme solution acidity can deactivate certain enzymes. The catalyst works in both basic and neutral conditions, and operates best under the highly acidic conditions encountered in fuel cells.
The study demonstrates a bottom-up approach based on understanding enzymes' functions and adding the components that perform these functions to a simple synthetic catalyst.
"Our methodology is broadly applicable," said Dr. Wendy Shaw, a PNNL biophysical chemist who led the study. "As we increase our understanding of how the outer coordination sphere functions, we can take our bottom-up approach to other catalysts to improve their reactivity, operation, specificity, and all of the things that enzymes do so well."
What's Next: To understand the structural role of the guanidinium pairing in the proton channel, the team is studying how the environment alters the catalyst's behavior and the impact of other structural elements. In addition, they are collaborating with two organizations to attach the catalyst to carbon nanotubes, for use in fuel cells, and to attach it to quantum dots for photocatalysis applications.
Acknowledgments:
Sponsors: This work was funded by the Office of Science Early Career Research Program through the U.S. Department of Energy's Office of Basic Energy Sciences (AD, WJS), and the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy's Office of Basic Energy Sciences (JASR).
Research Area: Chemical Sciences
User Facility: EMSL
Research Team: Arnab Dutta, John A. S. Roberts, and Wendy Shaw, PNNL
Reference: Dutta A, JAS Roberts, and WJ Shaw. 2014. "Arginine Containing Ligands Enhance H2 Oxidation Catalyst Performance." Angewandte Chemie International Edition. Communications. DOI: 10.1002/anie.201402304
Related Highlights:
Adding Natural Elements to Synthetic Catalysts Speeds Hydrogen Production
Proton Delivery and Removal Can Speed or Distract Common Catalyst
