Predicting Potholes on the Road to Renewable Energy
Storing wind-generated electricity as fuels means catalysts must avoid low energy states
(May 2014)

To create fast catalysts that avoid dangerous and delaying potholes, scientists built a scientific model that they can use to map the road a catalyst will take. The model predicts and allows scientists to avoid unnecessary stops or detours. Enlarge Image.
Image provided by Frontiers in Energy Research -- the EFRC Newsletter. Sign up today.
Results: When it comes to driving reactions to store electrons from wind turbines in the chemical bonds of use-any-time fuels, the path the catalyst takes matters. Researchers want to avoid low-energy and high-energy catalytic intermediates that could either represent a thermodynamic sink (even stopping the reaction) or require adding excessive amounts of energy to reach the final products. Scientists at the Center for Molecular Electrocatalysis (CME) devised a new computation-based method to predict the intermediates. The Center is an Energy Frontier Research Center led by Pacific Northwest National Laboratory (PNNL) and funded by the U.S. Department of Energy's Office of Basic Energy Sciences.
"Our goal is to avoid bottlenecks," said Dr. Simone Raugei, a PNNL theorist who led the study. "To make the reactions fast and efficient, we need to take out those undesirable intermediates."
Why It Matters: Cooling and heating our homes and offices with renewable energy would reduce environmental issues of fossil fuel use. However, wind and solar energy fluctuate greatly depending on the time of day and the location of the turbines or solar stations. One option is to store the generated electrons in chemical bonds. Making these bonds and then breaking them to get the electrons back to do work requires catalysts. This study provides scientists with an important tool in designing catalysts that work as quickly and efficiently as possible.
Methods: Using computational resources at EMSL and the National Energy Research Scientific Computing Center, the team devised a new computation-based method to calculate free energy maps, which show the energy required or given off in each step of the reaction. They tested the new method against nickel-based catalysts with a pendant amine in the second coordination sphere of the metal center; these catalysts were designed and characterized by scientists at the Center. The catalysts can either split hydrogen gas into protons and electrons or produce hydrogen from protons and electrons. The team input three easily accessible data points: redox potentials for the Ni(II)/Ni(I) and Ni(I)/Ni(0) couples of the nonprotonated complex, as well as the pKa or proton donating ability of the parent primary aminium ion.
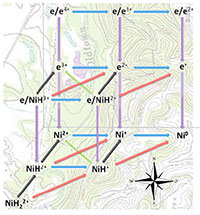
The result was an accurate prediction of the thermodynamics of intermediates as well as information on the reduction potentials, hydride donor abilities, and pKa values of both the protonated nickel center and the pendant amine. In addition to predictability under the normal conditions, the model allows for predictions at high temperatures and extreme pressures. While this method was built specifically for the hydrogen-specific catalytic system, the strategy is amenable to almost any catalytic system.
What's Next? This work continues to be implemented in new catalyst design in the CME. The catalyst team has begun work on a complementary model to fill in some of the other potential bottlenecks to complete the energy landscape.
Acknowledgments:
Sponsor: This research was supported as part of the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences.
Research Team: Shentan Chen, Ming-Hsun Ho, R. Morris Bullock, Daniel L. DuBois, Michel Dupuis, Roger Rousseau, and Simone Raugei, PNNL
Research Area: Chemical Sciences
User Facilities: Computational resources were provided at EMSL at Pacific Northwest National Laboratory and the National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory
Reference: Chen, S, MH Ho, RM Bullock, DL DuBois, M Dupuis, R Rousseau, and S Raugei. 2014. "Computing Free Energy Landscapes: Application to Ni-based Electrocatalysts with Pendant Amines for H2 Production and Oxidation." ACS Catalysis 4:229-242. DOI: 10.1021/cs401104w
