A Sweet Approach to Carbon Nanospheres
(December 2005)
The remarkable transformation of naturally abundant carbohydrates including simple sugars to highly porous elemental carbon nanospheres readily occurs in aqueous solutions that are heated just above the boiling temperature of water in a closed system. Under such conditions, these molecules (made up of one part carbon and one part water) actually dehydrate even though they are dissolved in water.
Magnetic resonance and in situ light scattering measurements have been used to probe the dehydration dynamics, yielding molecular clues about the process by which the spheres form. In the initial stages of dehydration, the evolving microscopic carbon spheres spontaneously assemble in a manner analogous to that by which a detergent emulsifies a mixture of oil and water. Subsequent loss of water by these assemblies leads to further coalescence of microscopic spheres to larger spheres thereby generating a grain-like structure with attendant interconnected porosity.
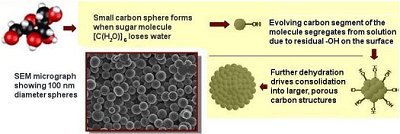
This highly regular and porous form of carbon can be isolated with sphere diameters ranging from tens of nanometers to tens of micrometers depending upon processing conditions. Ease of preparation, chemical robustness, and resident hierarchical porosity are attractive for applications ranging from sequestration of contaminants in air or waste streams, as catalyst supports for petrochemical production, and as electrically conducting phases in energy conversion devices including fuel cells.
This research was done by PNNL scientists Gregory J. Exarhos, Yongsoon Shin, and Li-Qiong Wang and by Brown University graduate student Chunhua Yao.
