Hydrogen Storage in Ammonia Borane
(May 2005)
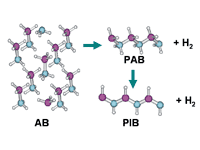
Ammonia borane releases a hydrogen molecule, forming polyaminoborane. Release of a second hydrogen molecule leaves polyiminoborane.
One of the major challenges in developing a fuel cell powered vehicle is safely storing sufficient quantities of the hydrogen fuel, without compromising customer expectations for performance and driving range. Furthermore, the hydrogen must be released from the storage system with minimal energy input, moderate temperature and pressure.
A team of PNNL researchers have been evaluating ammonia borane, the inorganic analog of ethane, as a potential hydrogen storage material. Ammonia borane, is a solid at room temperature composed of 19 weight percent hydrogen. Unfortunately, the bulk ammonia borane may release hydrogen too slowly for use as a hydrogen storage media for an automobile. However, the multidisciplinary PNNL group have been studying the impact of nanoscaling on the kinetics of hydrogen release. They have observed when ammonia borane is put into the pores of a mesoporous silica, with a pore diameter of 6.5 nanometer, the rate of H2 release increased by nearly 2 orders of magnitude.
One of the next challenges the researchers are addressing is to optimize the quantity of stored hydrogen and to understand how to make the process reversible. This work will continue as part of the DOE Center of Excellence in Chemical Hydrogen Storage.
The research team for this effort includes Tom Autrey, Maciej Gutowski, Anna Gutowska, Liyu Li, Yongsoon Shin, Chongmin Wang, Shari Li, John C. Linehan, R. Scott Smith, Bruce D. Kay, Benjamin Schmid, Nancy Hess and Wendy Shaw.
