
New Lab-Funded Initiative in Chemical Transformations
By Karl Mueller
The Chemical Transformations Initiative has been launched at Pacific Northwest National Laboratory! The CTI is funded through the Lab-Directed Research and Development program as a lab-level initiative to develop foundational capabilities at the national laboratory for chemical conversion processes having minimal or no environmental impact.
To minimize impact, we must develop the thermodynamics, kinetics, and reactor engineering of heterogeneous electrocatalysis and acid-base catalysis required to intensify converting waste carbon into higher energy and higher value products. Transportation sectors that require high energy density—aviation, shipping, and heavy trucking—will then gain local access to high-value fuels with minimal environmental disturbance. The constraints on carbon will require the processes to operate in highly distributed "mini-refineries" that must operate at low temperature and pressure, while being capable of handling complex reactants. Read more.
Energy Everywhere—A Big Idea Evolution
By Robert Weber

Feedstocks for renewable fuels have evolved over the past two decades in response to economic, social justice, and environmental demands associated with diverting huge quantities of resources needed to substitute even small fractions of our annual demand for petroleum. The changes have also reflected our increasing command over technology available for converting feedstocks that are not particularly energy dense into gasoline-like and diesel-like fuels. Yet, the renewable fuels industry is still nascent, reliant on government subsidies rather than supply/demand economics, for sustained profitability. Subsidies and mandates are still required because the feedstocks are expensive and difficult to convert, and the market does not yet sufficiently value sustainability. Read more.
The Contradictory Catalyst
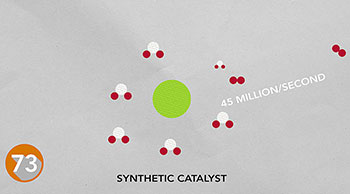 One reason we can't bottle summer sunshine and save the solar energy for rainy days is that we don't have an efficient way to store it. Nature stores energy in chemical bonds. Researchers are trying to design catalysts based on inexpensive metals to store energy like nature does.
One reason we can't bottle summer sunshine and save the solar energy for rainy days is that we don't have an efficient way to store it. Nature stores energy in chemical bonds. Researchers are trying to design catalysts based on inexpensive metals to store energy like nature does.
The chemical bonds in hydrogen gas, for example, could power fuel cells, internal combustion engines or generators. Using a natural catalyst from bacteria for inspiration, researchers have developed the fastest synthetic catalyst for hydrogen production -- producing 45 million molecules per second. Instead of a costly metal, this catalyst uses inexpensive, abundant nickel at its busy core. Read more.
90 Seconds of Discovery:
How to Sustainably Fuel a Hydrogen Fuel Cell
 To make sustainable hydrogen fuel cells for your car or your home, we need to produce hydrogen fuel from a source other than fossil fuel. PNNL chemist Molly O'Hagan explains in 90 seconds. Watch this video and learn more.
To make sustainable hydrogen fuel cells for your car or your home, we need to produce hydrogen fuel from a source other than fossil fuel. PNNL chemist Molly O'Hagan explains in 90 seconds. Watch this video and learn more.
January 2017
Catalysis News
DOE Calls PNNL "World Leader in Catalysis." The Department of Energy Office of Science released their evaluation of PNNL. They gave high marks to PNNL's science and technology. Read more.
A Cooperative Way to Make Ammonia. In new work, researchers discovered vital information about the manner in which the bacterial enzyme produces ammonia. Read more.
Lin Named Among World's Most Influential Scientists. Yuehe Lin is included on the 2016 Highly Cited Researcher list. Read more.
Light Strikes Gold to Create Better Catalysts. Tiny particles of gold are highly stable and have other attractive features for industrial uses. However, it's been difficult to control the size and shape of single-crystal nanostructures. Scientists revealed a special strategy that lets them synthesize a plethora of hexagonal or triangular crystals. Read more.
Bullock Selected as AAAS Fellow. Morris Bullock, Director of the Center for Molecular Electrocatalysis, was honored for his research. Read more.
PNNL to Partner with University of Oregon. A new agreement will allow scientists to obtain appointments that bridge the two research institutions. Read more.
If you have feedback – ideas, suggestions or questions – about IIC's Transformations, please contact Kristin Manke.