Hydrogen Storage: Operando Methods in Catalysis
(March 2006)
A major challenge in replacing petroleum-based fuels with hydrogen is safe storage of the hydrogen. One promising approach to hydrogen storage is in compounds of ammonia, boron, and hydrogen, generically NHxBHx. These materials have high theoretical volumetric and gravimetric capacities for hydrogen. Ammonia borane (NH3BH3) releases hydrogen upon heating to moderate temperatures in both the solid and liquid phase. More recently it has been proposed that metal nanoparticles enable the release of hydrogen at room temperature, however, little is known about the structure or mechanism of activation.
Researchers at PNNL studying the catalytic activation of hydrogen release from amine boranes using a combination of operando x-ray absorption fine structure (XAFS) spectroscopy and nuclear magnetic resonance (NMR) techniques. Operando spectroscopy permits the direct observation of key intermediates under reacting conditions, i.e., at reaction pressure and temperature, to gain insight into catalytic processes.
Operando XAFS spectroscopy, performed at the Advanced Photon Source at
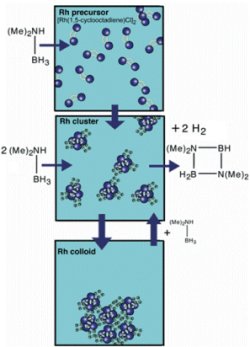
One of the crucial insights provided by Operando11B NMR spectroscopy is the formation of a short-lived amine borane intermediate. [Full Image]
Argonne National Laboratory, has enabled us to identify and characterize a cluster of four rhodium atoms that is a critical species involved in the catalytically-induced release of H2 from amine boranes. These results demonstrate that metallic nanoparticles are not directly involved in the catalyzed release of H2. Furthermore, this work shows that catalyst structure changes as a function of the concentration of reactants and products. These are important findings that will enable the design of concepts to control transformations in a multitude of related catalytic processes.
Operando 11B NMR spectroscopy was used to probe the evolution of the boron species to elucidate the mechanism of hydrogen release from the amine borane. Using NMR in concert with XAFS provides a complementary view of the catalysis pathways thus providing a more detailed understanding of how the four Rh atom cluster reacts with amine boranes. One of the crucial insights provided by 11B NMR is the formation of a short-lived amine borane intermediate, shown in the figure. These observations support a catalyst species that activates hydrogen release via an intramolecular pathway and not by the dehydrocoupling mechanism that is predominant in the non-catalyzed release of hydrogen.
Significance
We combined operando XAFS and NMR methods to elucidate the mechanism of hydrogen release from amine boranes. XAFS spectroscopy shows that the predominant rhodium species formed under the catalysis conditions is a Rh4 cluster (diameter of approximately 0.3 nm) rather than a 2 nm-diameter Rh nanoparticle. Knowledge of the Rh structures provides key insights into the catalysis species and the mechanism of the formation of hydrogen from amine borane compounds. We anticipate this class of Rh4 clusters may play an important role in both dehydrocoupling and hydrogenation reactions.
This research was done by Yongsheng Chen, John L. Fulton, John C. Linehan, and S. Thomas Autrey.
Reference: Chen Y, JL Fulton, JC Linehan, and T Autrey. 2005. "In-situ XAFS and NMR Study of Rhodium Catalyzed Dehydrogenation of Dimethylamine Borane." Journal of the American Chemical Society 127(10):3254-3255. DOI:10.1021/ja0437050.
