A Better Bit of Binding
New catalyst-free method decorates electrode with designer molecules for fuel cells, sensors
(December 2013)
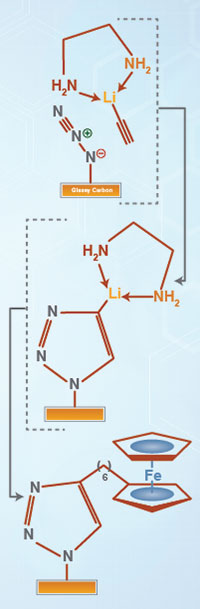
Scientists developed an efficient, flexible method of adding tailored molecules to a conductive carbon electrode. The team’s new method attaches a nitrogen-based molecule, reacts it with a triple-bonded lithium-based hydrocarbon, and swaps part of that molecule for the desired one. The method results in a variety of materials with possible uses in energy and security technologies. Flier | slide
Results: In studying how to modify carbon electrodes for catalyzed reactions, scientists at the Center for Molecular Electrocatalysis designed a process for creating a plethora of specialized electrodes at room temperature. The Center is an Energy Frontier Research Center, funded by the U.S. Department of Energy's Office of Basic Energy Sciences. Pacific Northwest National Laboratory leads the center. Today's reactions require metal catalysts or high temperatures and concentrated acids. In just three steps, the new method produces electrodes with the same coverage as traditional methods, but with less energy, in less time, and with no catalyst.
"Mild conditions allow better selectivity," said Dr. John A. S. Roberts, the chemist who led the study. "With aggressive conditions, the thing you want might happen, but other things you don't want might, as well."
The prestigious Chemical & Engineering News highlighted the study.
Why It Matters: Designing and perfecting efficient synthesis of revolutionary new forms of matter with tailored properties can change our nation's energy landscape. Fuel cells, longer-lasting batteries, and other energy challenges require materials with unique features. This new synthetic route provides the desired materials quickly and efficiently.
"The method provides interesting energy-related materials, but it is not specific to energy-related problems," said Roberts. "It could be useful in other areas where carbon material modifications are of interest, including sensors."
Methods: Starting with a glass-like carbon electrode, the team attached an azide group using iodine azide (IN3). The addition-elimination reaction replaces a hydrogen atom on the electrode (specifically, an atom on the edge of an sp2 carbon) with an azide. The team then added lithium acetylide-ethylenediamine, which reacts with the azide to form a 5-membered 1,2,3-triazolyl ring. This surface-attached group retains the lithium atom, providing an anchor point that reacts cleanly with a large variety of electrophilic coupling reagents including alkyl halides, organic carbonyl compounds such as esters and aldehydes, and silyl chlorides.
"If you look at the number of compounds in these categories, it is very, very large," said Roberts. "We now have dozens of functional groups to choose from, and we don't need a catalyst."
The team measured the resulting materials. Using voltammetry, they showed that the attachments were made. Taking X-ray photoelectron spectroscopy measurements by Mark Engelhard at DOE's EMSL, a scientific user facility at PNNL, the team determined the elemental composition of the surface. With these measurements, the team confirmed that the new method achieved the same coverage as the more conventional and less synthetically flexible copper-catalyzed reaction between azides and alkynes.
"We discovered a fairly general way of covalently binding things to conductive carbon materials," said Roberts.
What's Next? The team is following up on the research using the time-of-flight secondary ion mass spectrometer, also in EMSL, to study the process and resulting materials. They are also applying the new route to fabricate catalytically active materials with molecular-scale control over surface structures.
Acknowledgments:
Sponsor: The Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences
User Facility: EMSL
Research Area: Chemical Sciences
Research Team: Atanu K. Das, Mark H. Engelhard, R. Morris Bullock, and John A. S. Roberts, Pacific Northwest National Laboratory; Fei Liu, University of Wyoming
Reference: Das AK, MH Engelhard, F Liu RM Bullock, and JAS Roberts. 2013. "The Electrode as Organolithium Reagent: Catalyst-Free Covalent Attachment of Electrochemically Active Species to an Azide-Terminated Glassy Carbon Electrode Surface." Inorganic Chemistry 52(23):13674-13684. DOI: 10.1021/ic402247n
