Adventure Planning, Catalyst Style
A new approach shows the molecular consequences of everything from taking unnecessary detours to getting hopelessly lost
(February 2015)
Storing renewable energy in chemical bonds, such as the one between two hydrogen atoms, could turn wind, solar, and other intermittent sources into sustainable ones. The proposed approach will aid scientists in designing specialized catalysts that can do the job.
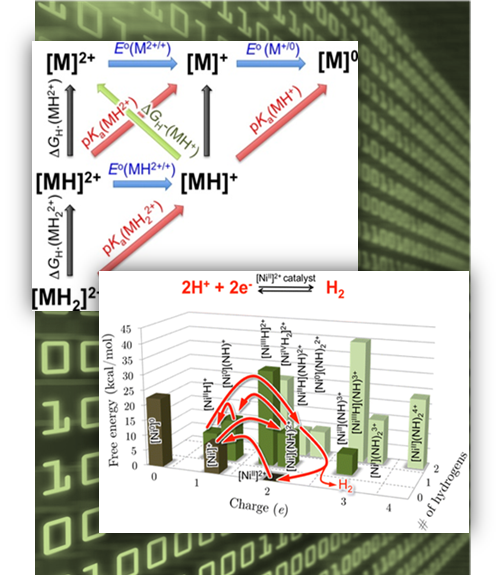
Results: Anyone who has planned a trip knows that some decisions have larger consequences than others. With catalysts, small design decisions can derail a trip through complex reaction paths. For example, packing in a potentially useful bit of molecular machinery could instead halt the reaction. At Pacific Northwest National Laboratory, scientists have elaborated on a strategy to map the catalytic route. Scientists can now explore design decisions with molecular catalysts that store or release energy from the chemical bond in dihydrogen (H2).
"Using it, we can modify one part of a catalyst and see how that affects everything, not just the optimal catalytic path but all the side reactions that can stop catalysis altogether," said Dr. Simone Raugei, a PNNL theorist who co-led the study. "We now know how catalysts with desired properties will behave in a given circumstance before we ever leave the computer. By working backwards, we can even ask which catalysts are the best performers for a set of conditions. We are on the verge of designing molecular electrocatalysts in silico -- or conducting research by means of computer modeling."
Why It Matters: Storing renewable energy in chemical bonds, such as the one between two hydrogen atoms, could turn wind, solar, and other intermittent sources into sustainable ones. Storing energy and releasing it on demand requires fast, efficient, affordable, and long-lasting electrocatalysts. Today's catalysts don't meet the mark. The proposed approach will help scientists design catalysts that do.
"Nobody has ever done research this comprehensive because we have not just the desired reaction pathway but all the undesirable ones as well," said Dr. Roger Rousseau, a PNNL chemist who co-led the project through much of its early stages.
Methods: Five years ago, the team began delving into the thermodynamic properties of hydrogen production and oxidation catalysts. They mapped the connections between different properties and the catalytic free energy landscape; that is, a chart of the chemical space accessible by the catalytic system.
With this information in their hands, they spent two years designing and validating, against in-house experimental measurements, equations that predict the free energetics of all the possible intermediate species that could arise in the family of electrocatalysts for H2. The proposed framework is very general and can be applied to other catalytic transformations, such as creating fuels from carbon dioxide, reducing oxygen (a critical reaction in fuel cell technologies), and reducing nitrogen to ammonia to make fertilizers.
"It is a matter of managing numerous variables and details. These go into simple informatics models that allow the computer to sort through all possible consequences," said Rousseau. "It is something computers can do very well."
Using a desktop computer, scientists can query the model about the thermodynamic properties needed to create the desired catalysts. They can use those parameters to inform experimentalists in their synthetic work. The experimentalists then can target complexes that have the desired set of properties.
This work was done at the Center for Molecular Electrocatalysis, an Energy Frontier Research Center. "A work of this breadth is only possible thanks to Energy Frontier Research Centers, which allow you to work in a truly multidisciplinary environment for a longer period of time to make breakthroughs," said Raugei.
The Center is led by Pacific Northwest National Laboratory and funded by the U.S. Department of Energy Office of Science's Office of Basic Energy Sciences.
What's Next? The PNNL team is adding a feature that lets the model provide structures and not just thermodynamic data.
Acknowledgments
Sponsor: Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science's Office of Basic Energy Sciences.
User Facilities: Computer resources were provided by the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility located at PNNL and sponsored by DOE's Office of Biological and Environmental Research, and by the National Energy Research Computing Center (NERSC) at the Lawrence Berkeley National Laboratory
Research Area: Chemical Sciences
Research Team: Simone Raugei, Daniel L. DuBois, Roger Rousseau, Shentan Chen, Ming-Hsun Ho, R. Morris Bullock, and Michel Dupuis, PNNL
Reference: Raugei S, DL DuBois, R Rousseau, S Chen, MH Ho, RM Bullock, and M Dupuis. 2015. "Towards Molecular Catalysts by Computer." Accounts of Chemical Research 48(2):248–255. DOI: 10.1021/ar500342g
