Mimicking Biology to Create Fast, Efficient Catalysts
Scientists are uncovering the design principles of natural catalysts, vital to energy storage
(May 2015)
Inspired by natural catalysts and bringing to bear scientific resources, researchers at Pacific Northwest National Laboratory track protons and electrons in the outer coordination sphere of catalysts. The result is faster and more efficient catalysts. One day, these catalysts could lead to processes that store intermittent renewable energy inside chemical bonds, holding the energy until it is needed and the bonds are broken. Artwork: Nathan Johnson Enlarge Image.
Pond scum is probably not the first thing that comes to find when you think about the future of energy storage. Yet, simple bacteria in the muck found in lakes and ponds pack electrical energy into chemical bonds and release that energy when it's needed. The bacteria create and break the bonds with the help of natural catalysts, or enzymes. The enzymes push the reaction, hurdling energy barriers, to make and break the bonds. Enzymes are typically fast, don't require excessive heat to jump start or maintain the reaction, and concentrate the essential building blocks. Enzymes work reversibly. In contrast, today's catalysts don't meet the mark.
But, the enzymes from pond scum's bacteria cannot simply replace industrial catalysts. Enzymes often slow or fail when their environment changes. And, as far as synthesizing the large and unwieldy molecules, the cost can be prohibitive, possibly costing more than using platinum or other rare metals.
"We want to capture the essential features," said Dr. Wendy Shaw, who leads the biomimetic catalysis research at DOE's Pacific Northwest National Laboratory. "We need to write the design guide for catalysts -- describe how to add just the best features."
To test their theories, Shaw and her coworkers have been mimicking a special type of enzyme, known as a hydrogenase. Catalysts inspired by hydrogenase could help solar and wind power overcome their on-again/off-again nature. Storing the energy, a.k.a. electrons, in batteries is an option, but stuffing the electrons into chemical bonds, as enzymes do, is another option with the potential for high-energy efficiency. Packing the electrons created on windy or sunny days into molecular hydrogen or dihydrogen (two hydrogen atoms bonded together) could be used to power fuel cells.
The reaction appears simple:
2 electrons + 2 protons = 1 hydrogen molecule
If everything is perfectly arranged and all of the conditions are optimal, it takes a catalyst 7 steps to create or break hydrogen. However, under most real-world conditions, it takes as many as 20 steps. This longer route includes possible dead ends that produce waste and diversions that produce long wait times. "The mechanism is remarkably complicated," said Dr. Morris Bullock, who leads the Center for Molecular Electrocatalysis, an Energy Frontier Research Center, based just down the hall from Shaw's labs. "There is a lot of detail in this process: taking the hydrogen apart, moving protons and electrons, and putting it back together."
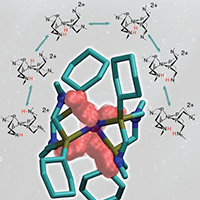
In breaking hydrogen (5 essential steps are shown here), ligands or ring structures move the protons to where they need to be. Copyright 2011: American Chemical Society
To work through the details, the team simultaneously studies enzymes' features and mimics those features in synthetic catalysts. Each approach informs the other and adds or deletes lines from the design guide, such as the right length for a channel to shuttle protons or the structure that focuses what the reaction needs.
Going beyond the active site. For years, conventional studies fixated on the heart of the catalyst, the active site, where hydrogen or energy was produced. The problem was that catalysts made from just an active site and thin coating of molecules didn't come close to Mother Nature's best.
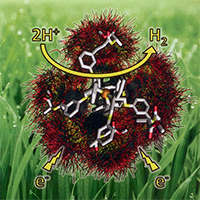
Scientists showed that adding a carbon-nitrogen bond in just the right spot on the catalyst's outer edge can cause the catalyst to work 5 times faster. ML Reback, B Ginovska-Pangovska, MH Ho, A Jain, TC Squire, S Raugei, JAS Roberts, and WJ Shaw: The Role of a Dipeptide Outer-Coordination Sphere on H2-Production Catalysts: Influence on Catalytic Rates and Electron Transfer. Chemistry: A European Journal. 2013. 19. Pages 1928-1941. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
While often dismissed, the protein scaffold -- as part of the outer coordination spheres -- appears to have a critical role in catalysis," said Dr. Aaron Appel, an organometallic chemist who focuses on basic energy research.
The team began experimental and computational modeling studies in 2004 to reveal the intricacies of the outer sphere, which folds around the active site. They found that the outer sphere acts like the best party planner. When the action starts, everything is just where it should be. As many protons as possible are available. The electrons are in the right spots. Countless details are handled smoothly with as little fuss or energy as possible.
But, it doesn't take much to gum up the works. Or help them along. Adding simple amino acids, the building blocks of enzymes, increases the speed and overpotential, or amount of energy needed to create hydrogen. Basically, the catalyst spits out hydrogen faster, but at greater cost. Moving the amino acid over slightly and binding it with another atom resulted in a more compact catalyst that could shuffle electrons faster. Of all the amino acids tested, adding acidic or basic groups resulted in the ability to concentrate substrates closer to the active site. As a result, the catalyst produced hydrogen as much as 5 times faster than without the changes.
As the researchers studied how different amino acids in different locations on the outer sphere affected the results, they kept running into the challenge of keeping the amino acids in their assigned location. Amino acids are very mobile because they are only linked at one end. The molecules must be precisely positioned to control the environment around the active site. The researchers took the first major step in getting the amino acids to stay put by attaching a small protein called a peptide platform to the catalyst. The peptide chain, known as a beta-hairpin, let the scientists clip amino acids into place. Adding the beta-hairpin is the foundation for future efforts in both the area around the active site and proton pathways.
At the heart of it. The team built these and other catalysts starting with a well-defined catalytic core known as the DuBois catalyst, which is named after catalysis leader and PNNL Fellow Dr. Daniel DuBois. The core mimics the function of a hydrogenase, making and breaking hydrogen. The team repeatedly uses this well-understood core, avoiding costly and time-consuming development.
The nickel-based DuBois catalyst is the result of years of experimental and modeling research done down the hall from Shaw in the Center for Molecular Electrocatalysis. Here, experts are unlocking the secrets of protons’ movement around the active site. The Center is one of 32 Energy Frontier Research Center, funded by the DOE Office of Science's Office of Basic Energy Sciences.
Proton paths. While the amino acid research on the DuBois-based catalysts provided insights into the outer sphere, researchers quickly focused in on the protons and their paths through the catalyst. Enzymes and synthetic catalysts use proton channels, built from one or more molecules, which pass the protons into and out of the catalyst, like runners in a relay race. Enzyme-inspired channels quickly proved beneficial. In a hydrogen oxidation catalyst, protons were able to quickly exit the active site. The result was speeding up the overall reaction. It also allowed the catalyst to twist into a form that significantly dropped the amount of energy needed to run and maintain the reaction. Experiments continued to showcase how building channels improved the reactions in different ways, but still the specific details needed for the design guide weren't there.
Getting those details and more involved walking down the hall to talk to their modeling colleagues.
"Modeling studies provide connections between behaviors -- important because it is impossible to probe all the interactions experimentally, especially in a larger outer sphere," said Bojana Ginovska-Pangovska, who performs modeling research at PNNL.
Designing proton pathways. Having determined the importance of proton movement, Shaw and her team began to build proton pathways to test the design principles. For example, by adding the amino acid glycine to a hydrogen oxidation catalyst, the scientists created a longer proton path. The catalyst had faster proton movement and improved efficiency. However, making the path longer by adding in a third relay made from the amino acid arginine didn't have the planned results.
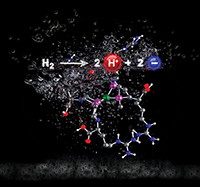
Modifying a hydrogen oxidation catalyst by creating a longer proton channel resulted in water solubility, enhanced proton movement, and an altered structure to allow create a catalyst that was six times faster than previously reported complexes. Enlarge Image.
The change in amino acid changed the active site's shape into a more efficient configuration. The result was a catalyst six times faster than previously reported complexes. At high levels of hydrogen, one molecule of the catalyst broke 144,000 molecules of hydrogen a second, orders of magnitude faster. In addition to the fast reaction rate, the overpotential was in the ballpark for similar catalysts, although still worse than enzymes.
While speed is important for use in fuel cells and other energy generation and storage uses, it means something different to scientists. "The high catalytic rate of this catalyst under high hydrogen pressure allowed us to identify the actual breaking of the chemical bond between the two hydrogen atoms as the rate-limiting event,” said Dr. Simone Raugei, a theorist part of the leading the computational effort of the program. “We are now in the unique position to design features to further improve the catalyst and make equally efficient at atmospheric pressure."
Changing shapes can also be valuable in catalysts. For example, when an enzyme's surroundings changes, such as when a cell becomes stressed by pollution, the enzyme folds over the active site, protecting it. When conditions improve, the enzyme flips open, like the lid of a box, and goes back to work. Shaw and her team tackled the triggers for this self-protection. Their initial work, in 2008, demonstrated that they could mimic this rudimentary control, triggering basic changes, causing the catalyst to solidify and fall out of solution.
"The enzymes are very precise, finely tuned machines. Mimicking their elegant operation is a challenge to say the least," said Ginovska-Pangovska.
Now, researchers are working to determine the mechanistic details of when and why the enzyme changes form. The ultimate goal is to shift in and out of reverse. Enzymes reversibly change hydrogen to protons and back, on the fly. By designing in proton channels and other features, the team created the first reversible catalyst, meaning that it can operate efficiently and fast in both directions, just like the enzyme. By altering the temperature and solvent where the catalyst resides, the catalyst could drive the reaction either way at each step: proton movement, hydrogen addition, and electron transfer. The result is a catalyst that can maintain fast rates in both directions.
"The resulting catalyst is reminiscent of the natural enzymes, operating at high rates with low energy requirement," said Dr. Arnab Dutta, a postdoctoral fellow who is bringing together the best of both worlds: natural enzymes and molecular catalysts "The catalyst needed the inclusion of the minimal and essential enzymatic features into the artificial catalyst to achieve this unprecedented behavior.”
Designing a future. Led by Shaw, researchers at PNNL have shown that the outer sphere plays a vital role in the success or failure of a catalyst. But, there is still a lot to do. The team is devising ways to place groups of atoms exactly where they are needed to get the desired catalysts. The beta-hairpin was a good start, but it allows more motion than an enzyme would. Now, the computational team is designing new molecular platforms where amino acids can be added and restricted just a bit, as enzymes do.
The team is also taking their successes in designing multitasking catalysts as a jumping-off point. They want to design a catalyst that can produce and break hydrogen molecules at room temperature and at rates that match or surpass enzymes. "We've added a lot of pages to the design guide for fast, efficient, and reversible catalysts," said Shaw. "And, that's due in part to our computational and experimental resources, but really it comes down to the people."
The power of the hallway. The research done over the past decade and continuing strong today draws on tools at PNNL and DOE user facilities, including EMSL and several light sources. "But, the real power comes in the form of a hallway, built half a century ago," said Dr. John Linehan, a chemist at PNNL who has worked along this hallway for more than 25 years.
The hallway is located in the Physical Sciences Laboratory at PNNL, and contains the offices and labs of experts in enzymes, synthesis, organic chemistry, computational chemistry, fuel cells, physics, organometallic chemistry, peptide chemistry, and nuclear magnetic resonance structure determination, to name a few. It means that all of these people have offices within a minute's walk of each other. Scientists drop by their colleagues' offices to discuss the latest results.
And, various groups meet frequently, sometimes every week and sometimes every day.
"It is really about getting experts into one room and building to solve problems bigger than one person could alone," said Shaw. "We can ask that difficult question. Look at it from a different perspective. The result? Solutions to very challenging problems begin to emerge, in ways that wouldn’t happen if we were working on our own."
Funding
- Department of Energy (DOE) Office of Basic Energy Sciences (BES): Early Career Award, Catalyst Biomimics: A novel approach in catalyst design, 2010-2015; Biological Principles of Energy Transduction: Basis for the Design and Synthesis of Hydrogen Catalysts, 2010-2013; Stimulus Sensitive Catalysis Research, 2008-Present
- Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by BES, 2009-2018
- PNNL Laboratory Directed Research and Development, Stimulus Controlled Catalysts, 2004-2006
Selected Publications
1. Arnab Dutta, Daniel L. DuBois, John A. S. Roberts, and Wendy J. Shaw, Amino acid modified Ni catalyst exhibits reversible H2 oxidation/production over a broad pH range at elevated temperatures. Proceedings of the National Academy of Sciences, 2014, 111, 16286-16291. DOI: 10.1073/pnas.1416381111
2. Bojana Ginovska-Pangovska, Arnab Dutta, Matthew L. Reback, John C. Linehan, Wendy J. Shaw, “Beyond the Active Site: The Impact of the Outer Coordination Sphere on Electrocatalysts for Hydrogen Production and Oxidation,” Accounts of Chemical Research, 2014, 47, 2621-2630. DOI: 10.1021/ar5001742
3. Arnab Dutta, Sheri Lense, John A. S. Roberts, Wendy J. Shaw, “Learning from Nature: Arg-Arg Pairing Enhances H2 Oxidation Catalyst,” Angewandte Chemie, 2014, 53, 6487-6491.
4. Matthew L. Reback, Garry W. Buchko, Brandon L. Kier, Bojana Ginovska-Pangovska, Yijia Xiong, John A.S. Roberts, Christina M. Sorensen, Simone Raugei, Thomas C. Squier, Wendy J. Shaw, “Enzyme Design from the Bottom-Up: An Active Nickel Electrocatalyst with a Peptide Outer Coordination Sphere.” Chemistry: A European Journal, 2014, 20, 1510-1514. DOI: 10.1002/chem.201303976
5. Arnab Dutta, Sheri Lense, Jianbo Hou, Mark H. Engelhard, John A. S. Roberts, Wendy J. Shaw, “Proton Channel Enables H2 Oxidation and Production for a Water-Soluble Ni Catalyst,” Journal of the American Chemical Society, 2013, 135, 18490-18496.
6. Sheri Lense, Arnab Dutta, John A. S. Roberts, Wendy J. Shaw, “A Proton Channel Allows a Hydrogen Oxidation Catalyst to Operate at a Moderate Overpotential with Water Acting as a Base.” Chemical Communications, 2014, 50 (7), 792-795.
7. Bojana Ginovska-Pangovska, Ming-Hsun Ho, Yuhui Cheng, Michel Dupuis, Simone Raugei, Wendy J. Shaw. “Molecular Dynamics Study of the Proposed Proton Transport Channels in [FeFe]-Hydrogenase.” Biochimica et Biophysica Acta:Bioenergetics, 2014, 1837, 131-138.
8. Matthew L. Reback, Bojana Ginovska-Pangovska, Ming-Hsun Ho, Avijita Jain, Thomas C. Squier, Simone Raugei, John A. S. Roberts, Wendy J. Shaw. “The Role of a Dipeptide Outer-Coordination Sphere on H2 Production Catalysts: Influence on Catalytic Rates and Electron Transfer.” Chemistry: A European Journal, 2013, 19, 1928-1941.
9. Sheri Lense, Ming-Hsun Ho, Shentan Chen, Avijita Jain, Simone Raugei, John C. Linehan, John Roberts, Aaron M. Appel, Wendy J. Shaw. “Investigating Steric and electronic Effects through the Addition of Amino Acid Esters, and the Use of Water as a Base, for Hydrogen Oxidation Catalysts.” Organometallics, 2012, 31, 6719-6731.
10. Avijita Jain, Garry W. Buchko, Matthew L. Reback, Molly O’Hagan, Bojana Ginovska-Pangovska, John C. Linehan, Wendy J. Shaw. “Active Hydrogenation Catalyst with a Structured, Peptide-Based Outer-Coordination Sphere.” ACS Catalysis, 2012, 2, 2114-2118.
11. Avijita Jain, Matthew L. Reback, Mary Lou Lindstrom, Colleen E. Thogerson, Monte L. Helm, Aaron M. Appel, Wendy J. Shaw. “Investigating the Role of the Outer-Coordination Sphere in [Ni(PPh2NPh-R2)2]2+ Hydrogenase Mimics.” Inorganic Chemistry, 2012, 51, 6592-6602.
12. Avijita Jain, Sheri Lense, John C. Linehan, Simone Raugei, Herman Cho, Daniel L. DuBois, Wendy J. Shaw. “Incorporating Peptides in the Outer-Coordination Sphere of Bioinspired Electrocatalysts for Hydrogen Production.” Inorganic Chemistry, 2011, 50, 4073-4085.
