Please Do Spill the Oxygen
Scientists show how clusters funnel atoms to create oxygen pools that benefit biofuels, fuel cells, and sensors
(September 2015)

Much like how tea flows through a leaky mug to pool under a table cloth, so oxygen atoms (red) spill out of a tiny cluster to pool under a graphene surface (blue). For the first time, scientists showed how the oxygen spills from the clusters to pool under graphene, providing valuable information for designing the next generation of catalysts, fuel cells, and sensors. Artwork by Ted Tanasse, PNNL Multimedia.
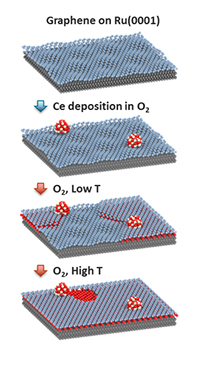
A graphene sheet on top of ruthenium metal has hills and valley because of their different atom spacing. Cerium oxide clusters (red and white) deposited on the surface fall into the surface's valleys. At low heat, the clusters slowly funnel oxygen under the graphene. There, the oxygen forms pools that flatten the graphene because of weaker graphene interactions. When enough oxygen is funneled through the graphene flattens out completely. At high heat, the graphene starts to etch and is consumed.
Much like the way tea flowing through a leaky mug pools under a tablecloth, so oxygen atoms flow out of a tiny cluster to pool under an extremely thin carbon sheet. This phenomenon, called oxygen spillover, is often discussed by those wanting to design catalysts to speed processes for biofuels, fuel cells, and sensors. Unfortunately, oxygen spillover is rarely seen. Until now. Scientists at Pacific Northwest National Laboratory and Karl-Franzens University in Austria clearly demonstrated how the oxygen spills.
"People talk about oxygen spillover all the time," said Dr. Zbynek Novotny, a PNNL chemist who worked on the study. "How it happens is not trivial to determine, and now, we can really see it and measure it."
Oxygen spillover could help solve a frustrating problem for those using the thin carbon sheets known as graphene. An inert material with desirable electronic and other properties, graphene provides a canvas on which to design a broad range of useful materials. Unfortunately, the graphene sheets do not like to interact with other materials. Here, the spilled oxygen atoms pool between the graphene and underlying metal and create an intermediate layer that smooths the otherwise rumpled graphene.
Why It Matters: New catalysts to produce fuels from plant matter could change the future of the nation's energy economy. A catalyst designed to quickly and efficiently promote the right set of reactions needs to have each task done in the right spot, at the right time, and as efficiently as possible. These catalysts need solid supports, just like a home needs the right foundation for the situation. The support on which to design the catalysts could be graphene. This study provides a key page in the design guidebook for catalysts as well as sensors and other devices.
Methods: Scientists Novotny and Dr. Zdenek Dohnálek along with Prof. Falko Netzer, one of the leading experts in two-dimensional oxides, laid down an atomically thin sheet of graphene on top of ruthenium metal. The resulting graphene surface has hills and valleys, because of the weak bonds that form between the metal and the graphene. So far, the same approach that has been used before.
Then, they added tiny clusters of a common metal oxide, popular in car exhaust systems, cerium oxide. The clusters settled on the top of the graphene surface. And then, the team turned up the heat, reaching temperatures typical for catalytic converters, around 600 to 750 Kelvin.
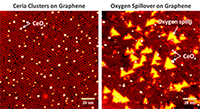
Using scanning tunneling microscopy in DOE's EMSL, scientists can see how ceria clusters settle onto a graphene surface (left) and begin pumping out oxygen atoms (larger yellow areas) under the graphene surface (right).
The team saw the oxygen atoms flow out of the cerium oxide clusters and slowly spread farther and farther away by combining high-resolution scanning tunneling microscopy with a conventional surface-sensitive technique, called Auger spectroscopy.
Specifically, the tools showed that the cerium oxide clusters passed their oxygen atoms through the graphene to the metal underneath. The cluster then pulled oxygen in from an oxygen-rich atmosphere, in a process that continued to cycle. The higher the temperature, the faster the layer of oxygen formed between the graphene and the metal. The oxygen layer weakened the bonds between the graphene and metal and flattened the graphene.
"When oxygen gets underneath, it changes the way graphene interacts with the metal," said Dohnálek.
What's Next? Seeing the oxygen spillover is an important step to designing new catalysts. The team is now modifying the graphene to create docking stations for catalytic clusters. These docks would let the team control where the clusters settled and the size of the clusters. The goal is to get cerium oxide clusters as small as a single cerium atom with two oxygens. These tiny clusters would add oxygen atoms more efficiently than larger clusters.
Acknowledgments
Sponsors: This work was supported by the DOE Office of Science's Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences
User Facility: EMSL
Research Team: Zbynek Novotny and Zdenek Dohnálek, Pacific Northwest National Laboratory; Falko P. Netzer, Karl-Franzens University, Graz, Austria
Reference: Novotny Z, FP Netzer, and Z Dohnálek. 2015. "Graphene/Ru(0001): Intercalation of Oxygen via Spillover." ACS Nano 9(2015):8617-8626. DOI: 10.1021/acsnano.5b03987
