Measuring Up: The Gold Standard for Catalysts in Real World Conditions
Combining 4 well-known reactions precisely predicts how well a catalyst performs
(April 2016)
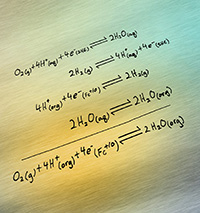
Using the first four chemical reactions, scientists calculated how well a catalyst could do at enabling the final reaction, which occurs in a carbon-based solvent. Artwork by Nathan Johnson, PNNL
High efficiency is the goal when using renewable energy to split water into hydrogen (a fuel) and oxygen, or when converting a fuel into electricity in a fuel cell. Catalysts are the workhorses that accomplish these conversions, but in some cases scientists haven't had an easy way to know if a catalyst is living up to its potential. Methods are well established for calculating that potential when the catalyst is in water, but not when in other solvents. Scientists have now found a way to bridge this gap. With just four reactions, the team showed how much energy each catalyst could use if it worked perfectly.
This work was done through the Center for Molecular Electrocatalysis, an Energy Frontier Research Center. The scientists are from DOE's Pacific Northwest National Laboratory (PNNL), University of Washington, and Yale University. The center is funded by DOE's Office of Science.
Why It Matters: A poorly performing catalyst can waste energy, and increase the cost and environmental impact. The challenge is determining if the catalyst is as energy efficient as it can be. Think of it this way: getting 30 miles to the gallon is good, unless you could have achieved 100 or more miles to the gallon. By determining the "gold standard" for the reaction, scientists can determine the wasted energy, or overpotential, of a catalyst for that reaction.
"This technique could let scientists weed out good catalysts from bad -- what would work and what would not," said Dr. Aaron Appel, who worked on the study at PNNL.
Methods: The team's method combines four well-known reactions to calculate the overpotential of a catalyst in a nonaqueous solvent. The reactions essentially start with the reaction in water, which is well known. Then, for each term in that first equation, they incorporate another equation that determines the energy difference between water and the desired solvent. Summing up the energies gives the final reaction in the desired solvent. Think of it like trading baseball cards. You start with what you have, and you make strategic trades to eventually get what you want.
Using this method, they determined the potential for turning oxygen into water in the common solvent acetonitrile is much higher that for the same reaction in another popular solvent, DMF.
The new method is quite general, and can also be applied to many other reactions, including the conversion of carbon dioxide to fuels. The result? An easy way to screen new catalysts for their energy use in a variety of reactions, letting scientists work with the best and avoid the rest.
What's Next? The team will be using the method to compare how catalysts enable the oxygen reduction reaction, which makes up half of fuel cells.
Acknowledgments
Sponsors: Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences
Research Area: Chemical Sciences
Research Team: Michael L. Pegis, Elizabeth A. Mader, and James M. Mayer, Yale University; John A. S. Roberts and Aaron M. Appel, Pacific Northwest National Laboratory; Derek J. Wasylenko, University of Washington
Reference: Pegis ML, JAS Roberts, DJ Wasylenko, EA Mader, AM Appel, and JM Mayer. 2015. "Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide." Inorganic Chemistry 54:11883-11888. DOI: 10.1021/acs.inorgchem.5b02136
