How to Heal Broken Bonds, Catalyst Style
Scientists show how to fix interior defects, possibly leading to a more stable and efficient catalyst for biofuel production
(June 2016)
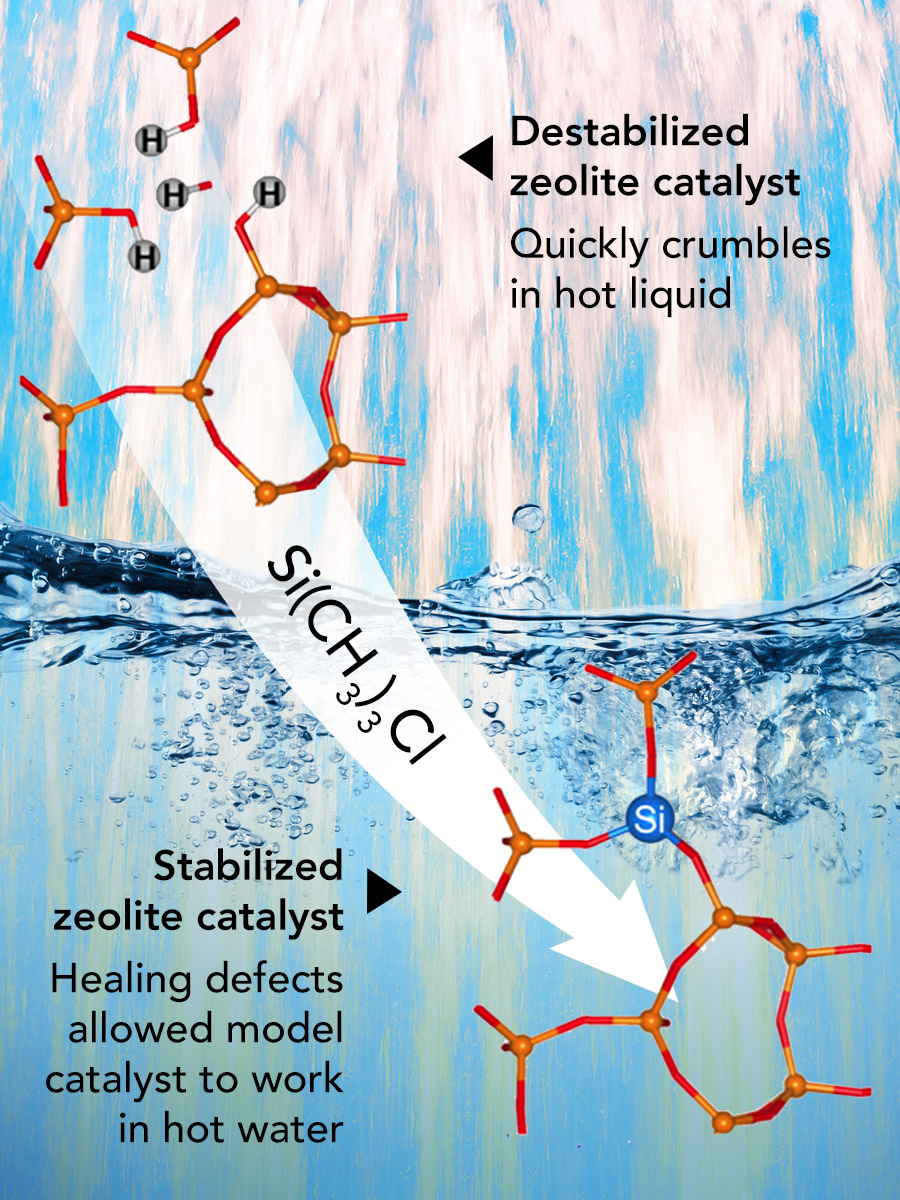
On the top, the broken bonds between silicon and oxygen atoms create an opening for water to destabilize the zeolite catalyst. On the bottom, the team’s approach adds in a silicon atom that stabilizes the catalyst by healing the defect. Modified by Nathan Johnson, PNNL, from referenced article (copyright 2016 American Chemical Society).
While popular catalysts called zeolites could help turn paper manufacturing waste and other biomass into fuel, the catalyst crumbles after just two days in hot water. And that's a problem because hot water is nearly ubiquitous in biofuel production. At Pacific Northwest National Laboratory, a team discovered that fixing broken bonds deep inside the material stabilized the catalyst and let it thrive in hot water.
"Rather than focusing on the surface, we tried to fix the inside," said Sebastian Prodinger, who worked on the experiments as part of his doctoral studies at TU München, Germany. "The reason is simple. The weak points are inside the crystal."
Why It Matters: Used extensively in industry today, zeolites are promising catalysts as part of efforts to turn biomass into transportation fuels. However, the stability of zeolites is challenging to understand and predict. This study offers insights to help design resilient zeolites, increasing their efficiency at producing fuels and chemicals.
"This research should lead to more resistant materials," said Dr. Miroslaw Derewinski, who mentored Prodinger and synthesized materials for the study. "We're finding ways to make the material better."
Methods: Zeolites have a sponge-like network of pores, or internal cavities, where reactions are catalyzed. Unlike sponges, these pores are "highly regular"; they are the same size and evenly spread across the material. The reaction's starting chemicals flow into the pores and the final products flow out. Zeolites can drive many different reactions such as transformations of single molecules or joining two molecules together.
This network of pores is made from silicon-oxygen-silicon and silicon-oxygen-aluminum bonds. These bonds form the material's superstructure, much like the framing of a house. When these bonds are broken, it creates defects. Water molecules filling the pores exploit these defects and cause the zeolites to crumble.
Prodinger and his colleagues wanted to prevent that damage from happening. Their first step was to synthesize zeolite samples riddled with defects, not on the surface, but inside the sample.
With the material synthesized, the team began to find ways to heal the defects inside the zeolite. They focused on the hydroxyl (-OH) groups at the defect sites and devised a way to rebuild the silicon-oxygen-silicon bonds.
"We had to modify the method to conform to PNNL safety requirements, which meant not working in gas tubes under high pressure," said Derewinski, a materials synthesis expert. "So we designed a flow reactor. It is far simpler approach and, now, can be used for other processes at PNNL."
To see if their approach worked, the team examined the healed material using nuclear magnetic resonance spectroscopy and other tools at PNNL's Physical Sciences Laboratory along with the helium ion microscope at EMSL, a U.S. Department of Energy Office of Science user facility.
The final product retained its porosity, even after days in hot liquid water. "This is a case where understanding why it fails is what you need to make it succeed," said Prodinger.
What's Next? Now, the team is applying the same healing approach to catalytically relevant zeolites. They're starting with a material that has fewer defects to see if their approach can close up defects and let the material work in hot liquid water. In addition, they are continuing to answer basic questions about the material. "We are working to get closer to real applications, and at the same time, we are working to understand why the sensitivities exist, how they are created, and how we can prevent them from happening," said Prodinger.
Acknowledgments
Sponsors: J.A.L. and A.V. provided discussion on the research; S.D.B. and I.A. performed detailed experiments using nuclear magnetic resonance spectroscopy and transmission electron microscopy, respectively. They were supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences. The Office of Science is the single largest supporter of basic energy research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information please visit http://science.energy.gov.
S.P. conducted the experiments with the assistance of experts in different techniques. M.A.D. provided data interpretation and guidance. Both were funded by the Materials Synthesis and Simulation Across Scales (MS3) Initiative conducted under the Laboratory Directed Research and Development Program at PNNL.
Research Areas: Chemical Sciences
User Facility: EMSL
Research Team: Sebastian Prodinger, Miroslaw A. Derewinski, Aleksei Vjunov, Sarah D. Burton, and Ilke Arslan, Pacific Northwest National Laboratory; Johannes A. Lercher, Pacific Northwest National Laboratory and TU München
Reference: Prodinger S, MA Derewinski, A Vjunov, SA Burton, I Arslan, and JA Lercher. 2016. "Improving Stability of Zeolites in Aqueous Phase via Selective Removal of Structural Defects." Journal of the American Chemical Society 138(13):4408-4415. DOI: 10.1021/jacs.5b12785
