Looking for a Cheap Catalyst
Scientists study how atoms behave when alcohol interacts with promising, inexpensive catalyst to produce hydrogen
(May 2008)
The energy involved in breaking different bonds in methanol is studied via computational models. Enlarged View
Results: One hurdle to producing hydrogen as an affordable, green alternative to gasoline is the exorbitant price of platinum, gold, and other precious metals used to catalyze or drive the reactions. So, scientists from Pacific Northwest National Laboratory's Institute for Integrated Catalysis and Southern Illinois University completed a theoretical study on how an inexpensive ceria catalyst can decompose a simple alcohol into hydrogen.
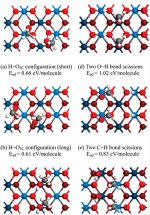
Methanol (CH3OH) contains four hydrogen atoms and can dissociate via several different reactions, depending on which bonds within the molecule are broken first. For example, if the C-O bond of breaks methanol first, undesirable byproducts and little hydrogen is produced.
Why it matters: As the financial and environmental costs of oil use continue to rise, cleaner-burning domestic fuels, such as hydrogen, are needed. To make manufacturing hydrogen a reality requires affordable catalysts, but first the atomic-level chemical and physical phenomena in the catalysts that drive the desired reactions must be understood.
Methods: The team showed that methanol molecules can attach through two different methods, molecularly and dissociatively, on two single crystal ceria catalyst surfaces. On both surfaces, breaking the hydrogen off the carbon of methanol produced the most stable products, but breaking the hydrogen from the oxygen atom was kinetically easier. Removing the hydrogen from the oxygen was almost spontaneous on one surface, known as the CeO2(111) surface. When the methanol coverage was increased, the O-H bond breaking became thermodynamically and kinetically favorable and resulted in more desirable hydrogen recovery.
To conduct this research, the team used the supercomputer and VASP software in the Department of Energy's Environmental Molecular Sciences Laboratory, a national scientific user facility at PNNL, and the National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory.
The results are consistent with earlier experiments, but explain the molecular details of dissociation that could not be observed experimentally.
What's next? The researchers plan on investigating the effect of defect sites on methanol decomposition over the ceria catalysts and compare with the experimental results under practical operating conditions.
Acknowledgments: The work was funded as a Laboratory Directed Research and Development project at PNNL. The computations were performed using the Molecular Science Computing Facility in EMSL. Computing time was made under a Computational Grand Challenge "Computational Catalysis." Part of the computing time was also granted by the National Energy Research Scientific Computing Center.
The research was conducted by Donghai Mei, N. Aaron Deskins, and Michel Dupuis of the Pacific Northwest National Laboratory, and Qingfeng Ge of Southern Illinois University. This work is part of PNNL's contributions to developing and deploying transformational tools and techniques for the biological, chemical, environmental, and physical sciences.
Citation: Mei D, NA Deskins, M Dupuis, and Q Ge. 2008. "Density Functional Theory Study of Methanol Decomposition on the CeO2(110) Surface." Journal of Physical Chemistry C 112(11):4257-4266.
