Meeting the Need for Catalytic Speed
Confining catalytically active hydronium ions inside zeolite catalysts enhances the rate of alcohol dehydration to hydrocarbons 100 times faster, paving the way to decentralized chemical processes
(September 2017)
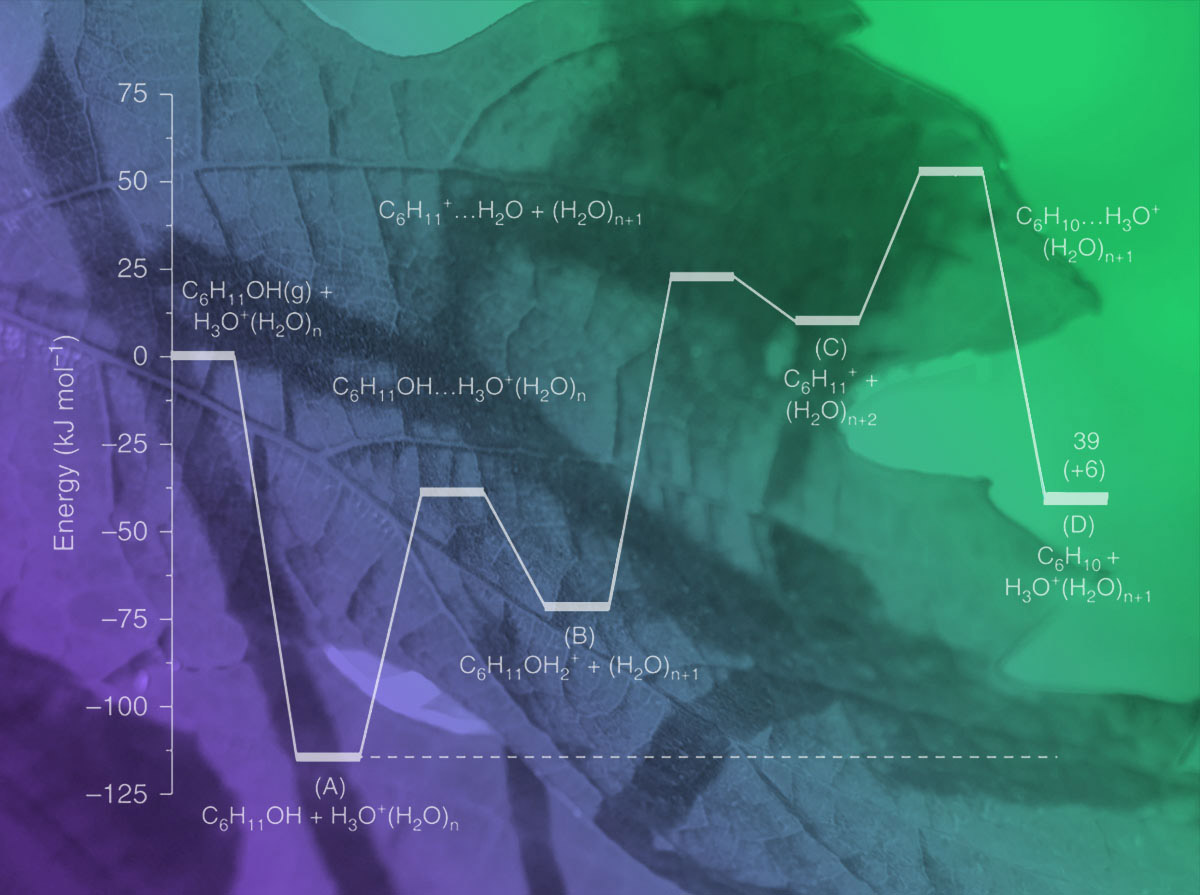 Converting agricultural waste into biofuels at low temperatures is too slow to be economically competitive. Scientists developed ways to accelerate the steps (shown here). Image provided by journal through Creative Commons arrangement; modified by Nathan Johnson, PNNL
Enlarge Image.
Converting agricultural waste into biofuels at low temperatures is too slow to be economically competitive. Scientists developed ways to accelerate the steps (shown here). Image provided by journal through Creative Commons arrangement; modified by Nathan Johnson, PNNL
Enlarge Image.
The rates to convert agricultural and other organic waste into biofuels at low temperatures are currently too low to be economically competitive. Led by Johannes Lercher, scientists at DOE's Pacific Northwest National Laboratory and TU München in Germany developed ways to accelerate the reaction rates. The solution was forcing a key reaction to occur in the nano-sized confining pores inside zeolites.
Why It Matters: Shipping organic waste to biorefineries over distances longer than 50 miles is costly and inefficient. Therefore, local decentralized conversion processes operating at lower than conventional temperatures are needed. The critical steps are related to the deconstruction of the organic waste, as well as to the elimination of oxygen from the intermediates by acid- base catalysis. This study provided a new approach to boost the reaction rates, showing a conceptual design to mitigate a critical step towards realizing decentralized fuel production.
"Theoretically, any municipality could produce its own fuel," said Lercher, Director of PNNL's Institute for Integrated Catalysis. "We, thus, aimed at designing new catalytic approaches to lay the foundations for the distributed production of energy carriers."
Methods: Natural catalysts, or enzymes, provided the inspiration for the new approach. Mimicking the small pockets in enzymes that help in accelerating highly specific catalyzed reactions, the team studied similar-sized cavities in zeolites that hosted hydrated hydronium ions. These hydronium ions catalyze the dehydration of alcohols, important intermediates in the conversion of biomass to hydrocarbon fuels.
The confined space of the zeolite pores forced the reactants together, so the hydronium ions have a greater chance of being close to an alcohol molecule than in an unconstrained solution. As the confines also stabilize intermediates, the reaction rates increase and can be realized at lower temperatures. In this way, the zeolite-catalyzed reactions were up to 100 times faster.
Cleared slide summarizing research conducted at Pacific Northwest National Laboratory. Download PowerPoint slide.
"The smaller the cavity, the larger the catalytic effect. We achieved the best results with diameters far below one nanometer," said Lercher.
What's Next? The researchers at the Institute for Integrated Catalysis are continuing to uncover the fundamental principles of zeolite catalysis. Lercher said, "We hope to use these to create the conditions required for new, decentralized chemical production processes that no longer require large-scale facilities."
Acknowledgments
Sponsors: U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences.
Research Area: Chemical Sciences
User Facilities: Portions of the nuclear magnetic resonance spectrometry experiments were done at the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE's Biological and Environmental Research located at Pacific Northwest National Laboratory. Portions of the computational work were performed using resources provided by EMSL and by the National Energy Research Scientific Computing Center (NERSC).
Research Team: Yuanshuai Liu, Sebastian Eckstein, and Eszter Baráth, TU München; Aleksei Vjunov, Hui Shi, Donald M. Camaioni, Donghai Mei, Pacific Northwest National Laboratory; Johannes A. Lercher, TU München and Pacific Northwest National Laboratory
Reference: Y Liu, A Vjunov, H Shi, S Eckstein, DM Camaioni, D Mei, E Baráth, and JA Lercher. 2017. "Enhancing the Catalytic Activity of Hydronium Ions through Constrained Environments." Nature Communications 8:14113. DOI: 10.1038/ncomms14113
