A Molecule Made to be Broken
Scientists can now control the rate of breaking and fixing dihydrogen molecule
(October 2017)
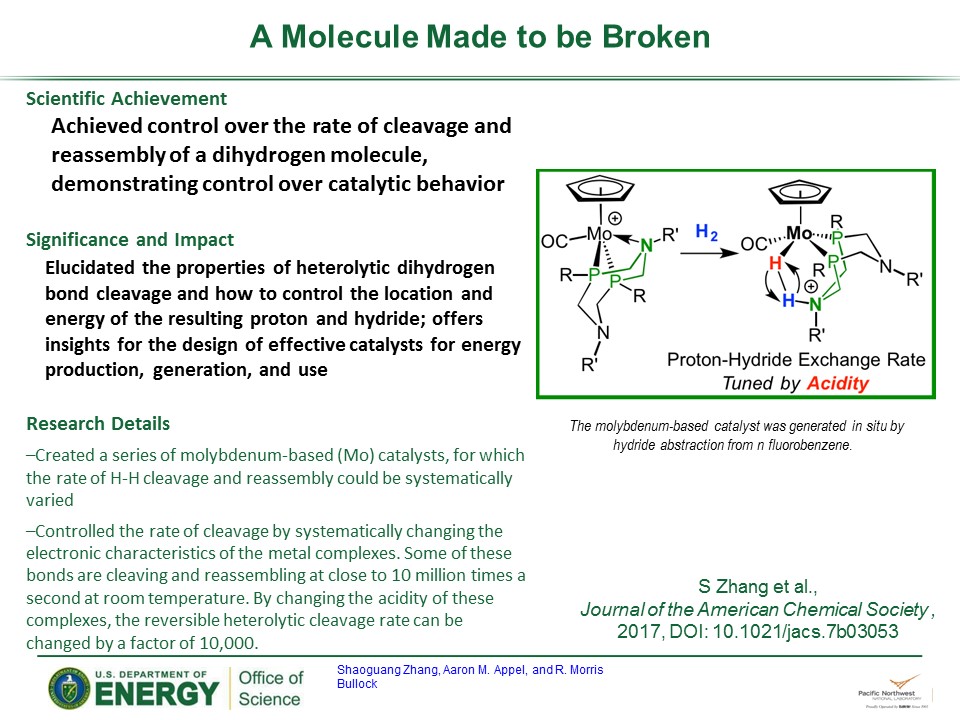
Hydrogen is the most abundant element in the universe. The dihydrogen molecule, with an H-H bond, is one of the simplest and most flexible in chemistry. Cleaving a dihydrogen bond to produce or store energy requires designing the catalyst with the perfect balance of properties to achieve the desired reactivity. In addition, the ability to get that molecule to reassemble itself and to control the rate of assembly and disassembly is important in the production of clean fuels. Morris Bullock and his colleagues at Pacific Northwest National Laboratory achieved control over the rate of cleavage and reassembly of a dihydrogen molecule.
Why It Matters: In the continual search for clean fuel production, scientists have been investigating simple ways to heterolytically cleave the hydrogen molecule into two uneven products. Understanding the properties of heterolytic dihydrogen bond cleavage and controlling the location and energy of the resulting proton and negatively charged hydride is important for the design of new catalysts for fuel cells and other clean energy sources.
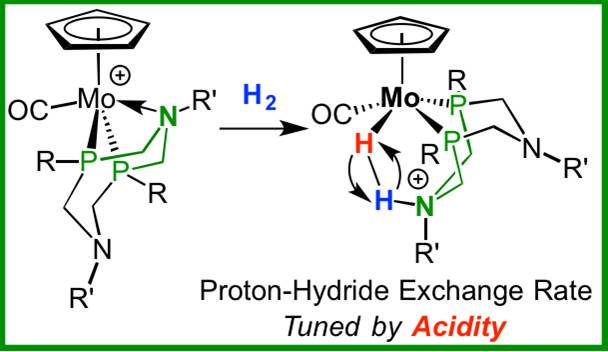
Methods: The dihydrogen bond is the simplest in chemistry but it offers a flexibility in how the bond is ruptured. It can be broken in two different ways, homolytically or heterolytically, into two identical fragments or two different charged fragments, a proton and hydride. Heterolytic cleavage is the breaking of the bonding electron pair into two uneven products. This is a common process in the use of hydrogen in fuel cells and in biological processes occurring in nature in which enzymes oxidize hydrogen. Reverse heterolytic cleavage is the process of taking these uneven fragments and reconstructing them to their original structure; that is, combining the proton and hydride and creating dihydrogen.
Before this study, Bullock and his colleagues investigated how dihydrogen bonds are broken and are reformed into a dihydrogen molecule. "What we're trying to do is find the right electronic characteristics so that the energy needed for cleavage is low," says Bullock, a catalysis scientist.
Designing this molecule is a balancing act. Earlier iterations of these molecules either were bonded too strongly to the catalyst after cleavage or were too weak to bond to the catalyst. In response, PNNL scientists created a series of molybdenum-based catalysts, for which the rate of H-H cleavage and reassembly could be systematically varied.
In addition, Bullock and his colleagues proved that a mechanism exists to control the rate of reversible heterolytic cleavage. Using nuclear magnetic resonance spectroscopy at PNNL, they observed the reaction as it occurred. Further, they controlled the rate of cleavage by systematically changing the electronic characteristics of the metal complexes. Some of these bonds are cleaving and reassembling at close to 10 million times a second at room temperature. By changing the acidity of these complexes, the reversible heterolytic cleavage rate can be changed by a factor of 10,000.
What's Next? Understanding the thermodynamic and kinetic properties of heterolytic dihydrogen bond cleavage and controlling the transfer of the proton and hydride are critically important for the design of new catalysts. The next step is determining how to achieve cleavage of the H-H bonds and control delivery of protons and hydrides after the H-H bond is broken.
Acknowledgments
Sponsor: U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences
Research Area: Chemical Sciences
Research Team: Shaoguang Zhang, Aaron M. Appel, and R. Morris Bullock, Pacific Northwest National Laboratory
Reference: Zhang S, AM Appel, and RM Bullock. 2017. “Reversible Heterolytic Cleavage of the H–H Bond by Molybdenum Complexes: Controlling the Dynamics of Exchange Between Proton and Hydride.” Journal of the American Chemical Society 139:7376-7387. DOI: 10.1021/jacs.7b03053