Quarterbacking Catalysts by Positioning Atoms
Single oxygen atoms are vital to designing better catalysts from the ground up
(March 2018)
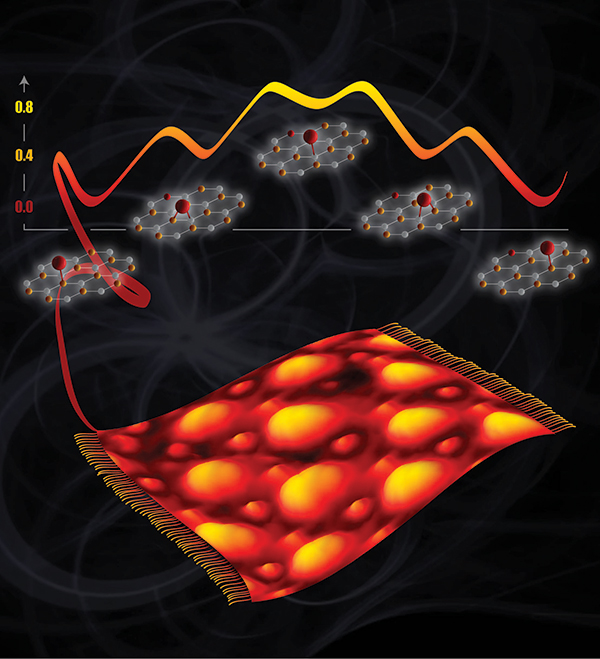
With a graphene sheet thrown like a carpet over the metal, oxygen atoms on top change their binding preference to only one carbon at a time instead of two. Image courtesy of Vanda Glezakou and Cortland Johnson, PNNL Enlarge Image.
To create a winning football team, quarterbacks send their team mates to the right spots. Positioned correctly, the players work around obstacles to drive the ball to the end zone. In much the same way, scientists position catalytic atoms to drive reactions that can yield fuels, plastics, or other desired products. A team led by Dr. Roger Rousseau and Dr. Zdenek Dohnálek at Pacific Northwest National Laboratory changed how scientists think about positioning key players: oxygen atoms. These atoms are vital in turning super-thin sheets of carbon, called graphene, into a unique catalytic support.
The team discovered that single oxygen atoms bind to graphene catalytic supports differently than expected. On a freestanding graphene sheet, oxygen most often rests between two carbon atoms. On a graphene sheet resting on a ruthenium metal support, single oxygen atoms bind to just one carbon. Moreover, because the graphene sheet buckles on the metal, these oxygen atoms appear only in certain predictable spots.
"This work let us understand oxygen binding at unprecedented levels," said Rousseau, a PNNL chemist who led the study's theoretical calculations. The team now knows exactly how oxygen atoms bind and the energy involved as well as the influence of the supporting materials.
Why It Matters: Creating faster and more efficient catalysts requires designing them from the bottom up. Scientists want to design the right structures to do the job rather than search among countless possibilities. To go back to our football analogy, the quarterback knows what needs to happen and designs the play to get the job done. That's designing the structure for the function.
This fundamental research shows scientists how to take advantage of precise spots on the graphene to build up model catalysts that can be faster and more efficient. "Oxygen atoms on graphene let us bind other groups," said Dr. Vanda Glezakou, a theorist on the study. "As a result, they make it possible to design precise and efficient catalytic arrays, essentially positioning the players where they can best work together."
"This research redefines what we know about oxygen binding to carbon atoms on metal-supported graphene, which is very important for their reactivity," said experiment lead Dohnálek, who holds a joint appointment with PNNL and Washington State University.
Summary: Beginning with a flat piece of ruthenium metal, the scientists grew graphene, a one-atom-thick layer of carbon. The two materials form a superstructure because the carbon and ruthenium atoms do not lay neatly on top of each other. This mismatch causes the graphene to pucker, forcing some carbon atoms to bind to the metal, while others don't. These differences in the graphene binding influence how oxygen atoms bind.
Having created this layered material, they delved into where the oxygen resided and how it behaved. They began with scanning tunneling microscopy. While the instrument is state of the art, the resolution wasn't sufficient to pinpoint the position of static oxygen atoms. So the team heated the material causing the oxygen atoms to move. How the oxygen moved told them about how it was bound.
Analyzing how and why the oxygen atoms moved required density functional theory calculations and massive simulations involving a thousand atoms. "The data analysis had to be really clever," said Rousseau. "This wasn't something anyone could do. The calculations were backbreaking."
The experiments and calculations required resources from two DOE Office of Science user facilities. The team used microscopes at the Environmental Molecular Sciences Laboratory in Washington State and supercomputers at the National Energy Research Scientific Computing Center in California.
By combining laboratory experiments and computational simulations, the team showed that single oxygen atoms bind preferentially to certain carbon atoms. Specifically, carbon atoms that are close to the underlying ruthenium but not bound to it. Less preferred sites for oxygen binding are between two carbon atoms; oxygens bound to carbon atoms that are, in turn, bound to ruthenium; and oxygens on untethered carbon atoms far from the ruthenium.
Having answered how oxygen behaves on carbon, the team is planning to use it as anchors to build model catalysts consisting of single metal atoms and small oxide clusters.
Acknowledgments
Sponsors: Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences (Z.N., M.T.N, V.A.G, R.R., Z.D.); an Alternate Sponsored Fellowship at Pacific Northwest National Laboratory (F.P.N.); and the University of Graz (F.P.N.)
User Facilities: EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL); National Energy Research Scientific Computing Center located at Lawrence Berkley National Laboratory.
Research Team: Falko P. Netzer, University of Graz; Zbynek Novotny, Manh-Thuong Nguyen, Vassiliki-Alexandra Glezakou, Roger Rousseau, and Zdenek Dohnálek, Pacific Northwest National Laboratory
Reference: Novotny Z, MT Nguyen, FP Netzer, VA Glezakou, R Rousseau, and Z Dohnalek. 2018. "Formation of Supported Graphene Oxide: Evidence for Enolate Species." Journal of the American Chemical Society. DOI: 10.1021/jacs.7b12791
Related Highlight
Tipping Water: Finding the Balance Between Keeping Molecules Whole or Splitting Them on Oxides
