Chromium's Crowning Achievement: Breaking Dinitrogen's Triple Bond
Catalyst forms ammonia precursors, valuable for fertilizers, plastics, and fuel
(April 2018)
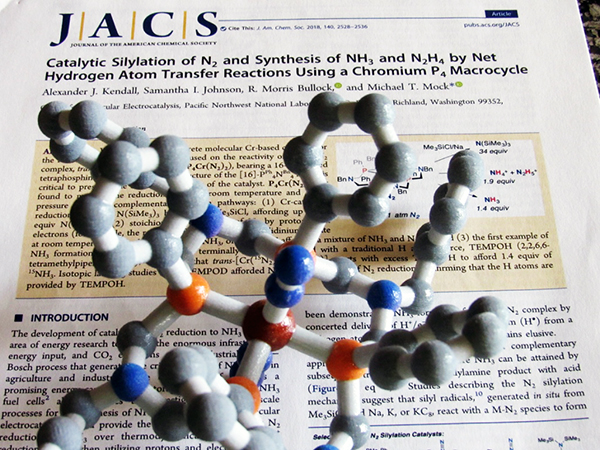
Supporting chromium (brown) inside a crown-like ligand resulted in a stable catalyst that breaks dinitrogen bonds at room temperatures and pressures. Image courtesy of Michael Mock, Pacific Northwest National Laboratory Enlarge Image.
For more than a century, industries have produced ammonia via an energy- and resource-intense process. It starts with dinitrogen (N2), which is about 80 percent of the air we breathe, two nitrogen atoms with an incredibly strong triple bond shared between them. The process gobbles up hydrogen from natural gas and releases carbon dioxide. Designing a better synthesis route for ammonia means understanding how the dinitrogen molecule interacts with and can be transformed by certain metals. Ideally, the process would use earth-abundant metals and renewable hydrogen sources instead of employing fossil fuels.
Led by Dr. Michael Mock at DOE's Pacific Northwest National Laboratory (PNNL), the team designed the first chromium catalyst for the job. Chromium is an earth-abundant metal, but unless it's supported by the right scaffold, it quickly fails as a catalyst for dinitrogen reduction. Placing chromium inside a crown-like support resulted in a stable catalyst that breaks dinitrogen bonds at room temperatures and pressures. Dr. Alexander Kendall performed the catalytic studies with the novel chromium complex that was a prior discovery in the dinitrogen reduction effort at PNNL.
"The field was wide open in terms of using chromium," said Mock. "Nobody had successfully developed a molecular chromium-based system for catalytic nitrogen reduction since the discovery of transition metal complexes containing dinitrogen over 50 years ago!"
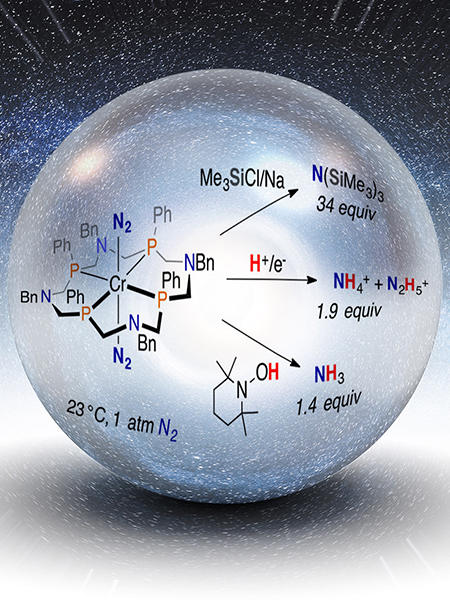
The chromium-based (Cr) compound, wrapped in a crown-like phosphorus-based (P) ligand, mediated three reactions that resulted in cleavage of the triple bond in dinitrogen (N2). Image courtesy of American Chemical Society and Nathan Johnson, Pacific Northwest National Laboratory Enlarge Image.
Why It Matters: This research offers a unique perspective into how chromium and other transition metals weaken the dinitrogen triple bond. This understanding is critical to reducing the amount of energy used to produce ammonia. It will also reduce the amount of natural gas (a hydrogen source) used and carbon released. Ammonia, for the manufacturing of fertilizers, is a vital part of food production. But it's also part of manufacturing plastics, fibers, explosives, and certain chemicals. Further, if ammonia synthesis could be made more economically, it could be used as a fuel. The fuel could be made using energy from wind turbines and solar cells.
Summary: Turning abundant dinitrogen into ammonia is vital for chemical manufacturing and large-scale farming. Creating a more efficient ammonia synthesis route means understanding the behavior of dinitrogen with transition metals that could serve as potential catalysts. Typically, chromium's low proclivity to interact with dinitrogen made it a challenging choice.
At PNNL, a serendipitous discovery led to insights about chromium's ability to transform dinitrogen into ammonia. Mock and his colleagues began by trying to synthesize a chromium-based compound surrounded by a supporting phosphorus-based ligand consisting of two 8-membered rings.
But chromium was being a sneaky metal. It rearranged the two rings into one large ring where chromium fit perfectly in the middle. Indeed, they had formed a continuous crown-shaped ligand that surrounds the chromium. After further investigation, they determined a 16-membered tetraphosphine macrocycle formed around the chromium. "It has the perfect combination of structural attributes that allowed us to study how chromium can mediate the reactivity of dinitrogen," said Mock.
The catalyst, officially known as trans-[Cr(N2)2(PPh4NBn4)] , is a rare example where Cr strongly holds on to dinitrogen. "We discovered three ways to make bonds to super-strong nitrogen bonds," said Dr. Samantha Johnson, a PNNL theoretical chemist who worked on the years-long study.
The first of the three pathways is catalytic silylation of N2 and the formation of a product known as N(SiMe3)3. This is the first discrete molecular chromium catalyst for nitrogen reduction. Of the metals similar to chromium on the Periodic Table, scientists have previously studied molecular molybdenum or tungsten complexes as players in the nitrogen arena. Both molybdenum and tungsten catalysts have been used successfully in dinitrogen silylation chemistry. The N(SiMe3) can be easily converted to an ammonia precursor (actually NH4+) by adding acid.
In the second path, dinitrogen is converted directly to NH4+ with the addition of protons and electrons from a chemical reductant, and depending on the reaction conditions hydrazine is also produced, a key component in rocket fuel. In this reaction, both the electron source and proton source must be present for the reaction to work. Using protons and electrons could lead to simpler ammonia production from renewable energy sources and water, a significant goal for the scientific community. "Although we are still working to make this reaction pathway catalytic, NH4+ is formed directly from N2 at room temperature and pressure," said Mock.
The final path uses a traditional organic reagent called "TEMPOH" that is known to donate hydrogen atoms. In a reaction with the chromium complex, gaseous ammonia is produced presumably by the net transfer of a hydrogen radical, which is a proton and electron bound together. "This was extremely unexpected, but incredibly exciting" said Johnson. "Typically, we would expect TEMPOH to be too weak of a hydrogen atom donor to complete this reaction."
The hydrogen atom results in the direct production of ammonia. "There have been other examples of ammonia formation with hydrogen atom sources, but in all cases the nitrogen-nitrogen triple bond has already been cleaved; this means you're reacting the hydrogen atom with a metal nitride," said Mock. "The significance is that we took a dinitrogen molecule with a triple bond intact and reduced it to ammonia."

Cleared slide summarizing research conducted at Pacific Northwest National Laboratory. Download PowerPoint slide.
At the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the Department of Energy's Office of Science, researchers are now investigating how the catalyst handles the reaction with protons and electrons supplied by an electrode, rather than TEMPOH or other chemical reductants. This is a key reaction in turning intermittent, renewable energy into easy-to-store fuels. They're also investigating the reaction in reverse, removing hydrogen from ammonia to produce dinitrogen.
Acknowledgments
Sponsor: Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the Department of Energy, Office of Science, Office of Basic Energy Sciences
User Facility: National Energy Research Scientific Computing Center, a Department of Energy Office of Science user facility located at Lawrence Berkeley National Laboratory
Research Team: Alexander J. Kendall, Samantha J. Johnson, R. Morris Bullock, and Michael T. Mock, Center for Molecular Electrocatalysis, Pacific Northwest National Laboratory
Reference: Kendall AJ, SI Johnson, RM Bullock, and MT Mock. 2018. "Catalytic Silylation of N2 and Synthesis of NH3 and N2H4 by Net Hydrogen Atom Transfer Reactions Using a Chromium P4 Macrocycle." Journal of the American Chemical Society 140:2528. DOI: 10.1021/jacs.7b11132
