Elusive Intermediates Captured for the First Time
New insights into catalyst aid in the hunt to produce hydrogen fuel
(February 2009)
Results: Expected but elusive—that's how scientists described two intermediates that may play an important role in the reaction that turns water into hydrogen. Now, thanks to the work of six researchers at Pacific Northwest National Laboratory, these intermediates are no longer a mystery. In a recent article featured on the cover of the Journal of Physical Chemistry C, the researchers provided images of two intermediates that occur during the reaction that changes water into hydrogen.
An intermediate is a transitory product created as a chemical reaction moves from its starting materials to the final product. Some reactions have a few intermediates, others hundreds.
"For a long time, people expected some intermediate should be formed during the reaction of oxygen and hydrogen on titanium dioxide," said Igor Lyubinestky, physicist at PNNL. "It was expected, but nobody had seen it." Until now.
Also, the team found that they could follow all of the steps that occur during oxygen and hydrogen transformation into water. "Forming water is much more complicated than thought," said Dr. Lyubinetsky.
Why It Matters: Discovering the intermediates and uncovering the intricate five-step process may shed light on the catalyzed reactions necessary to mass produce hydrogen. This simple molecule could power fuel cells that provide cleaner, quiet sources of energy.
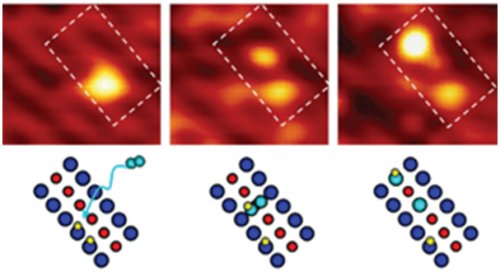
Two intermediates were discovered in the conversion of water to hydrogen (top: scanning tunneling microscope images). Forming these intermediates begins with oxygen (aqua). It snuggles in between the raised oxygen rows (blue), pulling a nearby hydrogen (yellow) on top of it. Later, this intermediate can split apart, creating oxygen and hydrogen (aqua and yellow) bound together and bound to a titanium atom (red).
Fuel cells are in use today, powering large buildings and tiny sensors and computers. Large stationary fuel cells provide backup power to hospitals, nursing homes, and other buildings. They also power remote outposts that do not have access to electricity. In vehicles, smaller cells are replacing or supplementing fossil fuel in cars, trucks, planes, trains, and boats.
Methods: The intricacy of the reactions and an exceptionally small number of molecules involved has prevented finding the intermediates until now. To combat these challenges, the PNNL team combined experimental techniques with theoretical techniques and computations.
The team prepared a partially hydroxylated, reduced titanium dioxide catalyst at room temperature so that the surface contained both hydroxyl groups and oxygen vacancies. Next, they exposed the catalyst to oxygen and recorded the reactions.
These reactions were imaged using the state-of-the-art scanning tunneling microscope at the Department of Energy's EMSL. This instrument, available to users from around the world, can show what single molecules and atoms are doing on catalytic surfaces.
Once the team's experimental experts acquired the images, the theorists went to work. They performed density-functional-theory calculations to help interpret the images. Based on the theory and experimentation, the team reported the first observed adsorbed hydroperoxyl. Hydroperoxyl is two oxygen atoms bound together and also containing a single hydrogen atom. The hydroperoxyl is attached to the surface, with a single bond forming between one of the oxygen atoms and a titanium atom.
The hydroperoxyl can then break apart. One of the oxygen atoms stays bound to the titanium atom. The remaining oxygen and hydrogen, still bound together, bounce down to bind to another titanium atom. This oxygen-hydrogen combination is the second elusive intermediate: a terminal hydroxyl group on the catalyst surface.
What's next? The team of three senior scientists and three postdoctoral fellows continues to explore catalysts and the reactions involved in producing hydrogen from water and sunlight. Their next paper, to be published soon in a prominent journal, looks at another variation, when the starting catalyst surface has oxygen adatoms.
Acknowledgments: DOE's Office of Basic Energy Sciences, Division of Chemical Sciences funded this research. The work, including use of the high-resolution scanning tunneling microscope and computational resources, was done at the DOE's Environmental Molecular Sciences Laboratory, a scientific user facility. Additional computational work was done at the National Energy Research Scientific Computing Center in Berkeley, California.
The research was done by Yingge Du, N. Aaron Deskins, Zhenrong Zhang, Zdenek Dohnalek, Michel Dupuis, and Igor Lyubinetsky at PNNL. This work is part of the Institute for Integrated Catalysis, a collaborative center for heterogeneous and molecular catalysis research and development.
Reference: Du Y, NA Deskins, Z Zhang, Z Dohnalek, M Dupuis, and I Lyubinetsky. 2009. "Imaging Consecutive Steps of O2 Reaction with Hydroxylated TiO2(110): Identification of HO2 and Terminal OH Intermediates." Journal of Physical Chemistry C 113(2):666-671.
