Dancing 'Adatoms' Help Chemists Understand How Water Molecules Split
The result might help understand chemical processes and catalysts for generating energy
(March 2009)
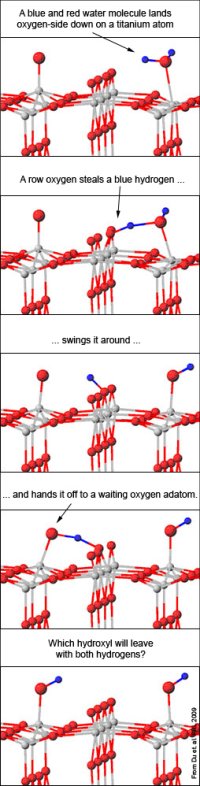
Results: Single oxygen atoms dancing on a metal oxide slab, glowing brighter here and dimmer there, have helped chemists better understand how water splits into oxygen and hydrogen. In the process, the scientists have visualized a chemical reaction that had previously only been talked about. The new work improves our understanding of the chemistry needed to generate hydrogen fuel from water or to clean contaminated water.
Why it matters: The scientists at Pacific Northwest National Laboratory made the discovery while trying to determine the basics of how the popular catalyst titanium dioxide breaks down water. The chemical reactions between water and oxygen are central to converting intermittent solar energy into hydrogen or other fuels that can be stored.
Acknowledgments: This research was done by Yingge Du, Aaron Deskins, Zhenrong Zhang, Zdenek Dohnalek, Michel Dupuis, and Igor Lyubinetsky at PNNL. They performed research using the scanning tunneling microscope and other instruments in the Department of Energy's EMSL, a national scientific user facility at PNNL.
The Department of Energy's Office of Science funded this research.
Reference: Du Y, NA Deskins, Z Zhang, Z Dohnálek, M Dupuis, and I Lyubinetsky. 2009. "Two Pathways for Water Interaction with Oxygen Adatoms on TiO2(110)." Physical Review Letters. DOI 10.1103/PhysRevLett.102.096102
For more information, see the PNNL news release.
