Inflating a Collapsed Organic Lattice
Molecular carbon host volume is increased by 26 percent
(January 2011)
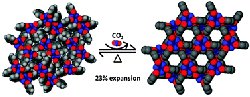
The conversion of a nonporous high-density phase (left) to a hexagonal porous phase (right) of the carbon host material was achieved with moderate pressures of CO2. This research was a “hot article” on Chemical Communications (November 2010). Enlarge Image
Results: Using pressurized carbon dioxide (CO2), scientists breathed new life into a collapsed host material by inducing a pore formation that can then sequester CO2. Like pumping up a flattened accordion, scientists at Pacific Northwest National Laboratory and the University of Missouri-Columbia revived the initial nonporous organic compound into a stable and porous host material. This transformation was produced by forcing the greenhouse gas into the lattice to create pores, increasing by 26 percent the surface area and potential host storage capacity. The scientists were able to watch in real time as the molecule opened and accepted CO2 as a guest molecule. This research has been identified as a "hot article" in the November 2010 issue of Chemical Communications.
Why It Matters: CO2 is a primary atmospheric pollutant from fossil fuel combustion in industry, vehicles and residential settings. Strategies for reducing CO2 will likely include methods for capturing and storing the gas before it is released to the atmosphere. Scientists are on the hunt for effective and efficient carbon capture and storage materials to reduce the amount of this pollutant in the atmosphere and reduce its greenhouse gas effects on climate change. This study points the way to obtaining a viable storage material for sequestering CO2.
Methods: With a formidable name, tris-o-phenylenedioxycyclotriphosphazene (TPP) is a simple chemical compound that can act as a host to different guest molecules. The scientists in this study investigated two guest-free forms of TPP. The high-density form is nonporous. The low-density form is nanoporous; that is, it has a regular porous structure. As a host lattice structure, TPP is valued for its ability to attract CO2 while at the same time repelling nitrogen.
The downfall of TPP is that its structure is easily disturbed by external stimuli, such as temperature, causing the material to structurally transform. When subjected to heat, this compound becomes highly unstable, collapsing when the guest molecule is removed. Once the compound has collapsed, it becomes nonporous and no longer useful as a host for gas storage.
In this new work, researchers subjected the high-density form of TPP to moderate gas pressure. The collapsed form then opened up and converted to the porous form. Researchers were able to monitor this lattice-swelling process in real time by means of in situ powder X-ray diffraction (PXRD) and high-pressure volumetric gas adsorption isotherms, a unique capability at PNNL. This gas-induced transformation opened up the host, creating an enlarged porous structure in which the CO2 gas could easily fit. The transformed structure appeared stable: once opened, it stayed open. However, the conversion of the collapsed form to the porous form of TPP is slow and pressure dependent. Thus, the formerly dense material was transformed into a useful gas storage material.
What’s Next: Researchers are looking at ways to expand the use of these materials for efficient CO2 sequestration at an accelerated rate. They plan to make a "smart material" by adding chemical groups that respond to incident light, magnetic field, or applied potential, leading to external control of the transformation that creates pores in the otherwise dense material.
Acknowledgments: This research was funded by the U.S. Department of Energy Office of Basic Energy Science and was performed by Jian Tian, Praveen Thallapally, Jun Liu, and Gregory J. Exarhos of Pacific Northwest National Laboratory, and Jerry L. Atwood of the University of Missouri-Columbia.
Reference: Tian J, P Thallapally, J Liu, GJ Exarhos, and JL Atwood. 2010. "Gas-induced solid state transformation of an organic lattice: from nonporous to nanoporous." Chemical Communications 2011 47:701-703. DOI:10.1039/C0CC04260A.
