A Molecular Makeover
Scientists see how tungsten trioxide transforms oxidizing alcohols into alkenes
(April 2008)
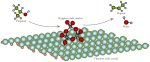
The research team determined the steps that occur when a catalyst helps split an alcohol, generating water and a carbon-based molecule known as an alkene. Enlarged View
Results: Trimming an alcohol into a smaller, more desirable molecule just got easier thanks to research at Pacific Northwest National Laboratory's Institute for Integrated Catalysis and the University of Texas at Austin. The team figured out the steps that occur when a catalyst helps split an alcohol, generating water and a carbon-based molecule known as an alkene. Tungsten trioxide was selected to fill the role of model catalyst, and propanol was used as the alcohol. Using the catalyst cut—in half—the energy required to drive the reaction.
Why it matters: Because catalysts lead reactions through lower-energy paths, their use can save large amounts of energy, which also prevents the pollution that would result from generating that energy. Catalysts have many applications in industry, such as converting petroleum products into plastics used for everything from carpets and capacitors to boxes, bags, and bottle tops. Understanding how tungsten trioxide works with propanol reveals characteristics that can be applied more generally to other catalytic systems.
Methods: At the Department of Energy's Environmental Molecular Sciences Laboratory, a national scientific user facility at PNNL, the researchers conducted a series of experiments to learn more about tungsten trioxide. First, they prepared clusters of tungsten trioxide by directly evaporating the tungsten trioxide onto a base of rutile titanium dioxide. This method produces identical clusters of tungsten trioxide.
Then, they tried different ways of combining the catalyst with very pure, specially marked propanol to see the effects of the different arrangements. These reactions were carried out in the ultra-high-vacuum molecular beam scattering chamber in EMSL and the results were measured using mass spectrometry.
For the first reaction, they deposited catalyst on a layer of propanol over a titanium oxide crystal base, and heated it, observing the formation of water and propene as the temperature climbed. Then, they deposited propanol on a layer of catalyst on the base and heated it. Finally, they heated catalyst on the base before the propanol was deposited on it, imitating the sustained heat that would occur in industrial production.
The experiments and subsequent calculations, including density functional theory calculations, showed that tungsten trioxide significantly lowers the amount of energy needed to convert propanol to water and propene.
The catalytic base does not affect the reaction significantly, an interesting result given that the base does affect other reactions catalyzed by tungsten trioxide.
What's next? The researchers plan similar experiments, replacing the tungsten trioxide catalyst with a similar one of molybdenum trioxide clusters. They also will try using a base different from titanium oxide, to reduce the attachment between the catalyst and the base.
Acknowledgments: Yu Kwon Kim at the University of Texas at Austin and Roger Rousseau, Bruce Kay, JM (Mike) White, and Zdenek Dohnálek at PNNL's Institute for Integrated Catalysis conducted this research.
The U.S. Department of Energy Office of Basic Energy Sciences, the Robert A. Welch Foundation, and the National Science Foundation funded the research.
Citation: Kim, YK, R Rousseau, BD Kay, JM White, and Z Dohnálek. 2008. "Catalytic Dehydration of 2-Propanol on (WO3)3 Clusters on TiO2(110)." Journal of the American Chemical Society 130(15):5059-5061.
