Researchers Improve Catalyst Efficiency for Clean Industries
(July 2016)
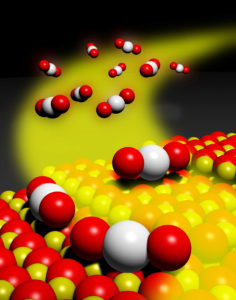
Mobile platinum oxide species trapped on a cerium oxide surface. The bonding of the platinum to surface oxygens creates isolated platinum atoms that are thermally stable and active for treatment of automotive exhaust pollutants.
Researchers have developed a way to use less platinum in chemical reactions commonly used in the clean energy, green chemicals and automotive industries, according to a paper published this week in Science.
Led by the University of New Mexico in collaboration with Washington State University, the research team developed a unique approach for trapping platinum atoms that improves the efficiency and stability of reactions. Find the abstract of the paper at http://science.sciencemag.org/cgi/doi/10.1126/science.aaf8800.
Using less of rare, costly platinum. Platinum is used as a catalyst in many clean energy systems, including in catalytic converters and fuel cells. The precious metal facilitates chemical reactions for many commonly used products and processes, such as converting poisonous carbon monoxide to less harmful carbon dioxide in catalytic converters.
Because of platinum's expense and scarcity, industries are continually looking to use less of it and to develop catalysts that more efficiently use individual platinum atoms in reactions. At high temperatures, however, the atoms become mobile and fly together into clumps, which reduces catalyst efficiency and performance. This is the primary reason catalytic converters are tested regularly for effectiveness.
"Precious metals are widely used in emission control, but there are always the issues of how to best utilize them and keep them stable,'' said Yong Wang, Voiland distinguished professor in the Gene and Linda Voiland School of Chemical Engineering and Bioengineering, Associate Director of the Institute for Integrated Catalysis at Pacific Northwest National Laboratory, and a co-author on the paper. "You want to use as little as possible to achieve your objectives, but it's normally hard to keep the atoms highly dispersed under working conditions."
Nanoscale trap captures platinum atoms. The research team developed a method to capture platinum atoms that keeps them stable and lets them continue their catalyzing activity. The scientists used a common, inexpensive manufacturing material, cerium oxide, to create a tiny, nanoscale trap. They shaped the cerium oxide into nanometer-sized rods and polyhedrons, which look like tiny pieces of rock candy, to capture the platinum atoms.
With their large surface areas and sufficiently high number of defects, the cerium oxide nanoshapes are able to capture the platinum atoms on their surfaces and keep them from clumping together, so the platinum can continue its work.
"The atom-trapping technique should be broadly applicable for preparing single-atom catalysts," said Abhaya Datye, a distinguished regents' professor of chemical and biological engineering at UNM who led the study. "It is remarkable that simply combining the ceria with a platinum catalyst was sufficient to allow trapping of the atoms and retaining the performance of the catalyst.
"Even more surprising is that the process of trapping occurs by heating the catalyst to high temperatures - precisely the conditions used for accelerated aging of these catalysts," he added.
Model for precious metal catalysts. Adding the cerium oxide to the catalyst is a simple process, too, with no exotic precursors needed.
"This work provides the guiding principles, so that industry can design catalysts to better utilize precious metals and keep them much more stable,'' said Wang.
The research is in keeping with WSU's Grand Challenges, a suite of research initiatives aimed at large societal issues. It is particularly relevant to the challenge of sustainable resources and its theme of energy.
Acknowledgments
Sponsors: This work was supported by a National Science Foundation (NSF) Grant Opportunities for Academic Liaison with Industry (GOALI) grant (J.J., H.X., S.R.C., A.K.D.), General Motors Global R&D (G.Q., S.O., M.H.W.), U.S. Department of Energy, Office of Science, Basic Energy Sciences (A.T.D., E.J.P., A.K.D., X.I.P.H., Y.W.), and the Center for Biorenewable Chemicals funded by NSF (H.X., H.P., A.K.D.).
Facility: This work made use of the JEOL JEM-ARM200CF at the University of Illinois at Chicago.
Research Area: Materials Sciences
Research Team: John Jones, Haifeng Xiong, Andrew T. DeLaRiva, Eric J. Peterson, Hien Pham, Sivakumar R. Challa, and Abhaya K. Datye, University of New Mexico; Gongshin Qi, Se Oh, Michelle H. Wiebenga, General Motors Global R&D; Xavier Isidro Pereira Hernández, Washington State University; Yong Wang, Washington State University and Pacific Northwest National Laboratory
Reference: Jones J, H Xiong, AT DeLaRiva, EJ Peterson, H Pham, SR Challa, G Qi, S Oh, MH Wiebenga, XIP Hernandez, Y Wang, AK Datye. 2016. "Thermally Stable Singe-Atom Platinum-on-Ceria Catalysts via Atom Tapping." Science 353(6295):150-154. DOI: 10.1126/science.aaf8800
This is a reprint of a press release written by Tina Hilding, Washington State University Voiland College of Engineering and Architecture
