Biofuels from Waste
Small amounts of carbon are sequestered in biomass such as waste from forestry operations, cropland, animal agriculture, and even sewage treatment plants. Recycling this underutilized carbon as renewable biofuels, experts say, could replace 25 percent of petroleum-based energy in the United States.
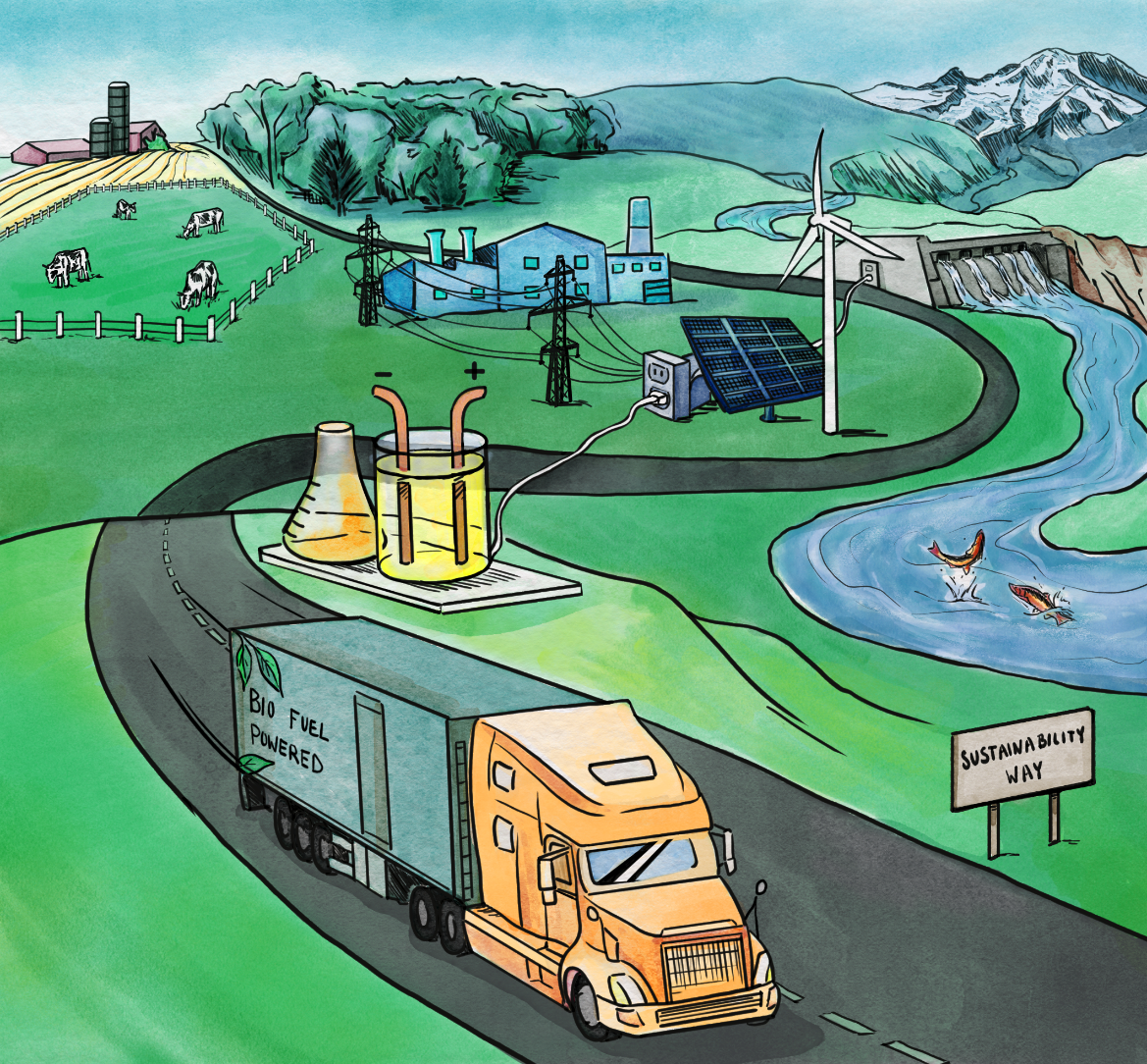
One goal of the Chemical Transformations Initiative is to develop portable technologies that use electrocatalysis to make fuel right where the biomass is available. PNNL graphic is courtesy of Rose Perry, Juan Lopez-Ruiz, Oliver Y. Gutiérrez, and Jamie D. Holladay.
To that end, by way of innovative chemistry, Pacific Northwest National Laboratory (PNNL) and its 2017-2020 Chemical Transformations Initiative (CTI) are developing catalysts that can help transform stranded carbon sources into high-energy liquid biofuels.
The same catalysts operate at low temperatures and pressures―a savings in energy and a boost to safety.
To make the carbon more efficient to get at, CTI scientists envision portable technologies that use electrocatalysis to make fuel right where the biomass is available.
"There are plenty of biomass sources, but the derived carbon amount is small―not enough to justify a (large-scale) conversion plant," said chemist and CTI researcher Oliver Y. Gutiérrez, who leads CTI's electrocatalytic hydrogenation research. "We need to create real breakthrough, disruptive technologies."
As an added benefit to sustainability, the electricity for these small biomass-conversion stations based on electrocatalysis would come from local solar, wind, marine, and other renewable power sources.
Catalytic Hurdles
The catalysts needed for these disruptive technologies present problems.
Processing of fuels such as petroleum at a large scale requires solid catalysts that operate best at extremely high temperatures and pressures. Electrocatalysis, on the other hand, operate in milder conditions to convert molecules that are dissolved in water and react to the surface of a submerged electrode.
To get this chemistry right requires the kind of fundamental research CTI is doing.
"There are certainly a lot of reactions that happen with liquids instead of gases on solid catalysts, but compared to gases, we don't understand them very well," said CTI collaborator and University of Washington professor Charles T. Campbell, whose research for the initiative focuses on fundamental reaction kinetics.
Investigating the atomic-level surface of a catalytic solid while in liquid has not been attempted until recently. There are valuable empirical studies out there, said Campbell, but it's better to combine detailed experiments and kinetic modeling with theoretical calulations.
CTI researchers have done that.
The pH Factor: Speeding Up Catalysis in Water
A recent research paper from this CTI collaboration, which appeared in ACS Catalysis, delivers insights on the influence of pH on hydrogenation, a process important to electrocatalysis.

University of Washington professor Charles T. Campbell does some of the molecular-level catalyst research for the Chemical Transformations Initiative. Photo is courtesy of the University of Washington.
Campbell, senior author of the study, explained that the paper introduces a theoretical kinetic model that is "a pioneering demonstration. It's an important step forward, just in terms of computational approaches to understanding."
The paper's lead author, Nirala Singh, was a postdoctoral researcher at both PNNL and the University of Washington, where he had a fellowship at the Clean Energy Institute. Still affiliated with CTI, Singh is now an assistant professor of Chemical Engineering at the University of Michigan.
The research demonstrated the influence of pH on aromatic substrates during aqueous-phase hydrogenation. Decreasing the pH from 8 to 1 increases the rate of hydrogenation—in this case, of phenol.
"We showed that we could change the reactivity of the hydrogen atoms on the surface so they do their chemistry more rapidly and aggressively," said Campbell. "Eight times more aggressively."
An Atomic Key to Better Fuels
In hydrogenation, molecular hydrogen (H2) reacts with other compounds in the presence of a catalyst. It's critical to any form of catalysis. For one, it reduces organic compounds and makes reactions possible at lower temperatures.
When hydrogenating catalytically, the reactant is always H2. But when the catalysis occurs electrochemically, the reactant is the sum of protons in a solution and electrons from the electrode.
The goal is to get the hydrogen ions in solution and the electrical voltage from the electrochemical cell to transform the hydrogen in water into hydrogen atoms on the surface of platinum or some other catalytic medium.
Those atoms are the key to better fuels―ones with more hydrogen and less oxygen.
In making fuel from biomass, said Gutiérrez, "it's hard to break the carbon-oxygen bonds to get rid of the oxygen and get higher fuel quality."
That gets to CTI's ultimate goal of using electricity from renewable resources to derive better fuel from biomass, he said. "The hardest part of that transformation is getting rid of the oxygen and adding hydrogen to the double bonds."
A 'Fruitful Collaboration'
Gutiérrez pointed to CTI's helpful diversity of disciplines, from Singh's modeling work to Campbell's expertise on how molecules react in the gas phase, which is "now being pushed into the environment of catalysis in water."
PNNL chemist Oliver Y. Gutiérrez takes the CTI research message to scientific audiences. Photo is courtesy of Oliver Y. Gutiérrez.
Acting CTI director Roger Rousseau and other computational scientists are adding to the research with theoretical models based on the actual dynamical water molecules.
"It's been a scientifically fruitful and synergistic collaboration, for sure," said Campbell of CTI. "We combined forces in a really good way to get new insight into the catalytic and electrocatalytic reactions that happen in water."
