Recycling carbon dioxide
By David Heldebrant
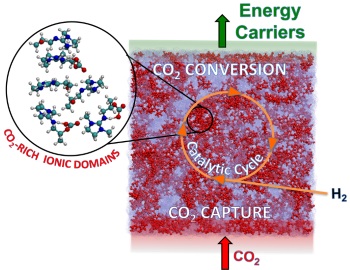
By performing carbon dioxide capture and conversion in the same solvent, we can use newly designed catalysts that help reduce how much energy it takes to turn carbon dioxide into an energy carrier or a chemical reagent.
Our power plants emit a lot of carbon dioxide when we produce electricity. An average 550 MW coal-fired power plant puts out about a million pounds of carbon dioxide per hour. These power plants emit so much carbon dioxide to the atmosphere that this heat-trapping gas is slowly warming our planet like a greenhouse.
We need strategies to reduce the amount of carbon dioxide in the atmosphere. Living plants—trees, grasses, shrubs—efficiently remove carbon dioxide from the atmosphere and sequester it into biomass using solar energy. How could we copy that ability?
This is where the IIC comes in. We hope to reduce carbon dioxide emissions from coal exhaust by combining the fundamental science of capturing the gas from exhaust streams and converting it into products normally made from crude oil, such as fuels and plastics. Recycling carbon dioxide this way lets us convert a waste gas into useful chemicals that improve our daily lives.
However, this process is not as easy as trees and bushes make it seem. Carbon dioxide capture and conversion are two energy intensive processes. To address this issue, I lead a BES early career program that investigates ways to combine these processes to improve the overall efficiency. My colleagues and I have found that the chemical transformations in capture and conversion are surprisingly similar. More intriguingly, carbon dioxide captured in solution forms carbonates—just as carbon dioxide in bubbly carbonated soda does—and this phenomenon allows carbon dioxide to be catalytically reduced even though it is negatively charged.
With this new fundamental knowledge, IIC researchers are exploring new, more energy efficient processes that recycle carbon dioxide. The high concentration of carbon dioxide in solution negates the need for high pressures commonly used for reduction, making the process more energy efficient. Our experiments showed for the first time that we could convert the carbon dioxide in the capture solvent directly. With these exciting results, we have started a series of new projects that take us to the next step, and a little beyond.
For example, the surprising reactivity of captured carbon dioxide inspired us to investigate less expensive transition metal catalysts. Typically, conversions occur in gas-phase reactions and use costlier conventional homogeneous catalysts based on ruthenium and rhodium. But the use of less-expensive catalysts can help drive down the costs associated with reusing carbon dioxide, needed for widespread adoption. Based on early experiments, we believe that the negatively charged carbon dioxide in solution might complex to heterogeneous catalysts, enabling for the first time these liquid-phase conversions of carbon dioxide. That insight, if it turns out to be correct, will help us design appropriate heterogeneous catalysts.
And beyond proof-of-concept, we're focusing on recycled products. My colleagues and I demonstrated recently that captured carbon dioxide can be catalytically co-polymerized with epoxides at atmospheric pressures to make polycarbonate plastics, a useful material that is used in eyewear, windshields and even jet planes. In addition, we are also focusing on controlling the reactivity of captured carbon dioxide to optimize the catalytic conversions to produce energy carriers such as fuels and high molecular weight polymers.
At the IIC, we are now poised to delve into these new areas of capture-conversion chemistry. It is important to note that our progress has been driven by the integration of strong applied, fundamental, and theoretical science within the IIC. Nearly every advancement we've made in the IIC has had components from all three approaches, with each providing insights that the other two then build from. This cycling of approaches, we hope, will lead us to the efficient recycling of carbon dioxide, ultimately reducing the amount of carbon dioxide we emit into the atmosphere.

