A Science Short: When a Catalyst Takes the Plunge
Water changes how cobalt-based molecule turns carbon dioxide into chemical feedstock
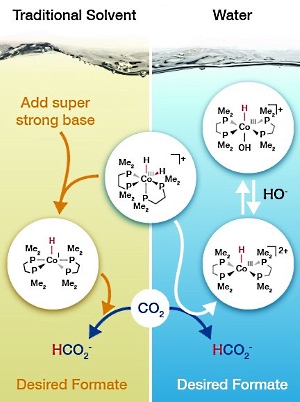
Catalysis researchers determined that the cobalt-based catalyst (center) takes a different path to adding hydrogen to carbon dioxide depending on whether it is in water or an organic solvent. Image: Nathan Johnson, PNNL
Imagine being able to use carbon dioxide, an inexpensive and abundant resource, to produce fuels and needed chemicals. The formulas involved aren't particularly complex, until you dig into the catalysts and conditions involved. Take adding hydrogen to carbon dioxide to create formate. This conversion has to overcome three challenges related to the choice of catalyst, solvent, and base.
Precious metal catalysts are effective but add to the cost. Earth-abundant catalysts, made from metals such as cobalt or nickel, can drive the reaction but require organic solvents that create waste disposal concerns. Add the need for a super strong base, and the reaction starts to look anything but simple.
Researchers at DOE's Pacific Northwest National Laboratory may have found a way around all three challenges by having a cobalt-based catalyst take the plunge into water. With some back-of-the-envelope calculations, Dr. Eric Wiedner and his colleagues predicted that a cobalt catalyst, (H)2CoIII(dmpe)2+ (dmpe=1,2-bis(dimethylphosphino)ethane), that worked in an organic solvent could take a different reaction path to work in water.
"By using thermodynamic predictors to design catalysts, we've avoided the shotgun approach and the associated time and cost," said Wiedner. "We understand how to control the catalyst thermodynamics, and now are able to extend our findings to design new catalytic systems."
Guided by the predictions, the team tested the catalyst in water using high-pressure nuclear magnetic resonance spectroscopy and a customized cell (see sidedebar). "This is a rare and powerful technique in the catalysis community," said Wiedner. The team's analysis demonstrated that the catalyst had worked just as predicted.
They found that with water as the solvent, the thermodynamics for the hydride transfer to carbon dioxide changed. This change eliminated the need for a strong organic base. Now, only sodium bicarbonate (baking soda) was needed.
Understanding how solvents, especially water, behave is crucial to designing the best catalysts. And the best catalysts are needed to transform carbon dioxide into a chemical that can serve as a building block for manufacturing fuels and chemicals.
This work was funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences.
Sidebar: PEEK Performance

The PEEK NMR tube is as simple to use as a standard glass NMR tube allowing routine observation of catalysts-in-action using high-pressure NMR.
Operando spectroscopy is the simultaneous collection of rate (how fast the catalyst works) and observation of the catalyst in action. Nuclear magnetic resonance (NMR) spectroscopy is one of the most useful spectroscopic techniques in organic and organometallic chemistry. The use of operando NMR spectroscopy to interrogate catalyst systems under pressure has been limited by the dearth of high-pressure NMR cells.
At PNNL, we have a long history of high-pressure NMR spectroscopy dating back to our fused-silica high-pressure NMR system in 1994. These cells could be safely operated up to 48,000 psi, but suffered from a very small cell volume (low sensitivity) and were cumbersome to use.
Striving for a more practical cell, we teamed with PNNL machinists to develop an inexpensive high-pressure NMR cell made from PEEK (polyether ether ketone) plastic in 2002. These tubes have large volumes, yield excellent multinuclear NMR results, are safely used up to 1,000 psi, and are extremely easy to use. Due to their safe and robust nature, we routinely use the PEEK cells for operando high-pressure NMR spectroscopy to understand how catalysts work during carbon dioxide hydrogenation.
The use of high-pressure PEEK cells for operando NMR studies sets PNNL apart from other research institutions and has allowed us to make difficult kinetic and mechanistic NMR studies routine and simple.

