Designing the Catalysts Needed for Sustainable Energy
by Dan DuBois
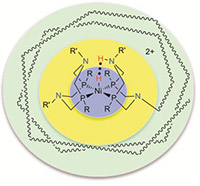 Schematic showing first (blue), second (yellow), and outer coordination spheres for an H2 production and oxidation molecular catalyst.
Schematic showing first (blue), second (yellow), and outer coordination spheres for an H2 production and oxidation molecular catalyst.
Electrocatalysts – which drive the interconversion of electricity and chemical energy in the form of chemical bonds – are key to storing and using energy generated by solar, wind, geothermal, or nuclear sources. To create these catalysts, the molecular electrocatalysis team at Pacific Northwest National Laboratory focuses on using three complementary principles:
- Take a modular approach
- Understand the results with modeling
- Control the movement of protons.
In the modular approach, catalyst structures are broken into first, second and outer coordination spheres or areas. The first sphere includes those atoms bound directly to the active metal center. This sphere can be used to control substrate or feedstock binding and thermodynamic properties. The second sphere consists of acids, bases and other functional groups near the metal that can interact directly with substrate molecules when they are bound to the metal. This sphere can facilitate substrate binding, bond formation and cleavage, and proton movement. The outer sphere constitutes the remainder of the catalytic system.
Once a general structural motif has been established for the coordination spheres, a second and equally important aspect is avoiding high-energy intermediates and thermodynamic sinks. For an energetically efficient catalyst, the free energy associated with the reaction must be close to zero. Catalysts that have very high-energy or very low-energy intermediates will be either slow, inefficient or both. Recently, simple models for predicting free energy landscapes for all intermediates in complete catalytic cycles have been developed for H2 electrocatalysts. These models will be invaluable for further improvements in catalyst design.
A third design principle is the control of the movement of protons. Because a proton is approximately 2000 times more massive than an electron, it is extremely important to provide low-energy pathways for the movement of protons between the metal center and acids or bases in solution. The presence of precisely placed acids or bases in the second and outer coordination spheres can provide this type of control. We now have numerous examples of catalysts in which the incorporation of proton relays reduces the overpotentials, or energy required, by more than 1 volt, and increases catalytic rates by more than three orders of magnitude.
These three principles constitute the scientific basis for our approach to the design of molecular electrocatalysts, which has led to some of the most active, selective, and energy-efficient catalysts based on nickel, cobalt, iron or manganese.A deeper understanding of these principles and how to apply them to catalyst development will lead to major advances in the development of fast, efficient, and durable molecular electrocatalysts. Such catalysts will have considerable impacts on our ability to use renewable energy sources and mitigate environmental problems associated with the use of fossil energy.

