
Getting Real with Catalysis Research
PNNL spectroscopic capabilities mimic harsh conditions of industrial operations
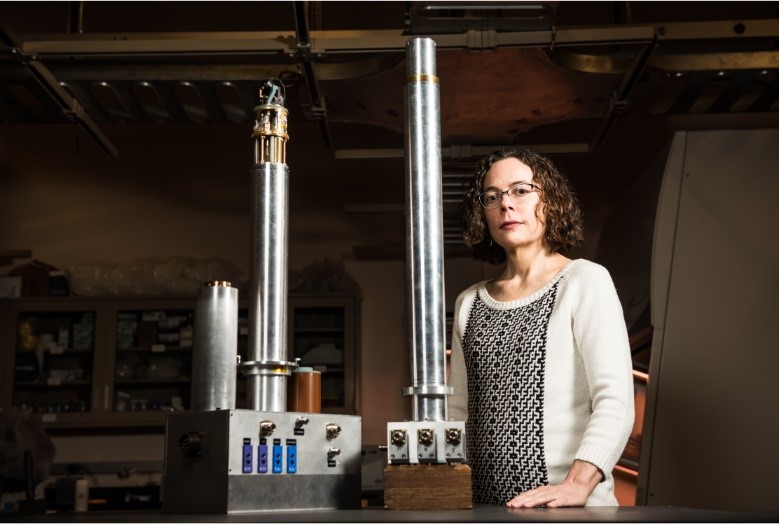
Nancy Washton, a PNNL chemist, oversees nuclear magnetic resonance spectrometers and related capabilities for the Environmental Molecular Sciences Laboratory. Scientists use a high-temperature NMR probe (left) that allows monitoring chemical reactions up to 350°C and a novel sealed NMR probe (right) for hazardous samples.
In 2018, Virgin Atlantic Airlines flew a maiden voyage with jet fuel made from recycled waste gas. This accomplishment, made possible by a unique catalyst developed at Pacific Northwest National Laboratory (PNNL), joins a host of other recent technological advances driven by fundamental discoveries in chemical science.
Catalysts promote chemical transformations by lowering the energy required to complete a reaction. To make a catalyst even more efficient, scientists need a molecular level understanding of the catalyst structure, reaction kinetics, intermediates, active centers, and pathways.
Within the Institute for Integrated Catalysis at PNNL, scientists use some of the world’s most sophisticated instruments to analyze and understand the elemental steps of catalytic reactions. These instruments include a suite of nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR) spectrometers.
With combined proton resonance frequencies ranging from 300 megahertz (MHz) to 850 MHz, the spectrometers allow scientists to study the structure and dynamics of solids, semisolids, liquids, or gases, alone or in combination. And with new technology designs, NMR experiments now mimic industrial operating scenarios–what scientists call "operando" or "in situ" conditions.
Where the Magic Happens

New rotor designs for MAS NMR include high-temperature materials and one-way valves that allow researchers to conduct catalysis experiments under conditions that mimic industrial operations.
Magic Angle Spinning (MAS) is a special kind of NMR that provides increased resolution spectra from solid samples. Its name refers to the specific angle–54.74 degrees–at which the sample rotor spins with respect to the NMR main magnetic field.
For many samples, this "magic" technique narrows the resonance lines, or peaks, to provide unprecedented spectral resolution for identifying more chemical species. The peaks also allow scientists to not only understand the chemical reactants and products, but also to uncover molecular structure at the active centers of catalysts.
With this fundamental knowledge, researchers can design new catalysts and catalytic synthesis routes that are faster and more selective. But they need confidence that their work will translate to real-world settings.
"To prove the reliability of MAS NMR for understanding catalysis operation," said Jian Zhi Hu, a PNNL chemist, "we need to show that it will generate similar values of critical experimental measurements, such as reaction activation energy and reaction rates, as those from a standard batch reactor on the benchtop."

Washington State University graduate student Nicholas Jaegers worked with Hu on in situ MAS NMR for the past four years for his doctoral dissertation. Jaegers' professor is Yong Wang, a PNNL-WSU joint appointee.
Two types of rotors now offer researchers the ability to conduct their experiments under more realistic conditions.
Hu led the design of a new, perfectly sealed rotor made of high-strength ceramic except for a commercially available high temperature sealing O-ring and spin tip. The new rotor can withstand the stress of spinning at 1500 revolutions per second and it works with a dramatically reduced sample volume—as low as 20 microliters.
The improvement in speed at high magnetic field allows studies of a wide range of metal nuclei with drastically enhanced spectral resolution and sensitivity. The new design also significantly decreases proton background signals, so it can be used to directly interrogate samples with low proton levels.
Another design, led by PNNL chemists Eric Walter and David Hoyt, includes a built-in one-way valve. Air-sensitive samples can be prepacked in a glove box, allowing sequential gas additions when needed. This way, researchers can investigate samples as the catalytic systems evolve without reopening the sealed rotor.
Signals in the Silence
Atoms and molecules in many catalyst systems can have unpaired electrons, which can broaden the signal too much to be observed by NMR. Or, as Walter said, "the electrons obliterate other chemical signals."
Electron paramagnetic spin resonance, or EPR, can directly interrogate the unpaired electrons that are silent to NMR. This interrogation yields information not only about the atom or molecule that hosts the unpaired electrons, but also its immediate chemical and physical environment.
Complimentary Capabilities for Catalysis
With the new designs, MAS NMR can probe the atomic-level structure and molecular dynamics of a catalyst system ranging in size from 0.1 nanometers to about 1.0 nanometers over timescales of pico-seconds to days. And it can reveal reaction intermediates or transient species that typically only exist under real operational conditions.
The specialized MAS NMR sample holders can be utilized on commercial systems at any field strength (e.g., 300 MHz, 850 MHz, etc.). For solids and gases, accessible temperatures range from -25°C to 325°C. For a substance with liquid, solids, and gases, the temperature range starts at -25°C and goes up to 275°C. The pressure range is from sub-atmospheric pressure up to 275 atmospheres (atm) at 120°C, and up to 225 atm at 250°C. The MAS NMR sample holders can use sample sizes from 20 microliters to 450 microliters at various magnetic field strengths. These sizes enable studies of even very small quantities of catalysts, reactants, and products.
Meanwhile, EPR works with sample sizes of 1 milligram to 50 milligrams at pressures ranging from vacuum (1e10-5 atm) to 100 atm, and temperatures from -270°C to 400°C. Together, the MAS NMR and EPR allow realistic investigations of reaction kinetics and intermediates associated chemical reactions for a breadth of catalysts.
Most of the instruments are located at the Environmental Molecular Sciences Laboratory, a Department of Energy Office of Science user facility located on the PNNL campus. A few others are in PNNL's Process Science Laboratory and Radiochemical Processing Laboratory.
A new DOE research facility, the Energy Sciences Center, is slated to open in late 2021. It will modernize and consolidate a portion of PNNL's wide array of chemical transformation research efforts under one roof.
Who knows what catalyst discoveries the next decade will bring to our nation's energy landscape?
