Effective Use of Renewable Electricity for Making Renewable Fuels and Chemicals
The increasing availability of renewably generated electricity could change the way that chemicals and fuels are manufactured by substituting conventional, thermally activated processes with possibly more selective and certainly more highly distributable electrochemically activated processes. Renewable electricity, however, is not free electricity. If obtained in bulk through a Purchasing Power Agreement, renewable electricity can cost around1 0.025 $/kWh (= 0.0069 $/MJ). Converting carbon dioxide, CO2, back into a fuel, say methane, requires adding energy equal to or greater than the heat of combustion of the fuel (greater, if the process is irreversible). The higher heating value of methane is 55.50 MJ/kg, so if that energy were derived from renewable electricity, the cost would be 0.0069 $/MJ x 55.50 MJ/kg = 0.38 $/kg. However, that cost is about three times larger than the current price of methane ($3/million BTU = 0.14 $/kg). In fact, extrapolating from experience in the electrolysis of water to make hydrogen,2 the other costs of electrolytic conversion (for example, equipment, labor) make the total cost about six times larger than the cost of the conventionally produced product.
While much basic science might be learned about electrocatalysis by researching the reversion of CO2 into methane or other reduced products, there would be little economic appeal in an eventual process, given the conditions of the current market. Of course, one could target products other than methane, but none of those listed offers compelling economics.
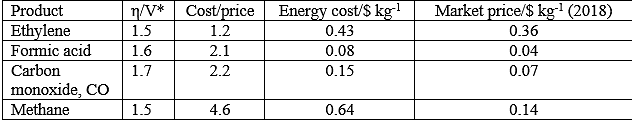
*This compilation of overpotentials was provided by National Renewable Energy Laboratory in a personal communication. The overpotential, η, is the voltage that must be applied to surmount kinetic barriers.
Alternately, one could start with a less oxidized feedstock and effect a less energy-consuming transformation, preferably one that results in a high-value product. That is the approach of both the Chemical Transformations Initiative (CTI) at Pacific Northwest National Laboratory (PNNL) and PNNL's contribution to the new Bioproducts Institute (BPI, a collaborative effort between PNNL and Washington State University). Both efforts are engaged in early stage research that targets practicable products as well as extrapolatable knowledge.
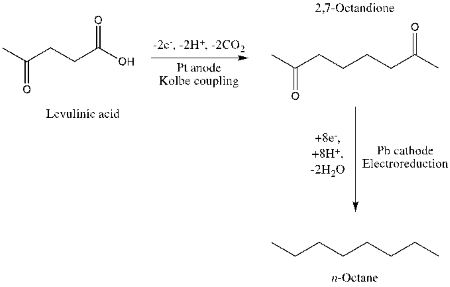
An example of the first aspect of that approach—starting with a less oxidized feedstock—can be found in work3 from the Institute of Environmental and Sustainable Chemistry, Technische Universität Braunschweig. They have electrochemically coupled levulinc acid and then electrochemically reduced the resulting diketone:
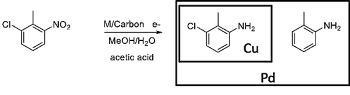
The second aspect, making a highly valued product, is exemplified by PNNL's collaboration with GSK, a global manufacturer of pharmaceuticals, to devise a continuous, electrochemical route to active pharmaceutical intermediates. This route is represented here by the conversion of a substituted nitroaryl into the corresponding aniline, the regio-selectivity of which depends on the metal used as the electrocatalyst and the applied potential:
Either aspect uses less electrical energy than reverting CO2 back into a fuel, but neither is going to make a significant reduction in the greenhouse gas footprint of a country as industrialized as the United States. Rather, the approaches of the CTI and the BPI target valuable products and effective use of byproducts from the processing of renewable or wasted carbon. Those approaches require deep experience and state-of-the-art facilities in the field of chemical catalysis, which is why both efforts are housed at PNNL in the Institute for Integrated Catalysis.
References
- US EPA, 2018, Green Power Pricing.
- National Renewable Energy Laboratory, 2009, Current (2009) State-of-the-Art Hydrogen Production Cost Estimate Using Water Electrolysis.
- P. Nilges, T.R. dos Santos, F. Harnisch, and U. Schröder. Energy Environ. Sci., Electrochemistry for biofuel generation: Electrochemical conversion of levulinic acid to octane, 2012, 5, 5231-5235, 10.1039/c1ee02685b.

About the author: Bob Weber is a senior scientist and the Sector Manager for Commercial Business in the Physical and Computational Science Directorate at Pacific Northwest National Laboratory. His research activities focus on heterogeneous catalysis for fuels and chemicals.

