The Top Five Things We've Learned in Creating Catalysts to Store and Release Energy
At the Center for Molecular Electrocatalysis, scientists discovered how controlled movement of protons can improve our ability to store electricity in chemical bonds
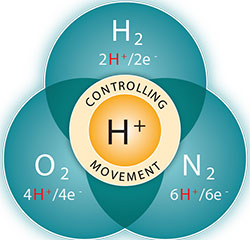
Reactions studied in the Center for Molecular Electrocatalysis include production of hydrogen and the reverse, oxidation of hydrogen, reduction of oxygen, and reduction of nitrogen to give ammonia.
Replacing fossil fuels with wind and solar energy requires a way to store the energy generated and release it when needed. Both of these renewable energy sources can contribute significantly to the nation's energy supply, but their energy output can vary over time periods as short as a few minutes to as long as a year. To overcome this variability demands energy storage.
One option is to store the energy in chemical bonds; that is, fuels. The necessary reactions demand efficient catalysts based on nickel or other abundant metals. Rational design of such catalysts, rather than relying on inefficient cook-and-look studies, means discovering and understanding the underlying scientific principles. Since 2009, researchers at the Center for Molecular Electrocatalysis (CME), an Energy Frontier Research Center funded by the DOE Office of Science Office of Basic Energy Sciences, have answered fundamental questions about what makes catalysts work. Their top five answers are...
1. Proton movement plays a vital role in the interconversion of electricity and fuels. Often overlooked in favor of studying the smaller, swifter electron, the movement of protons within the catalyst, or from the solution to the catalyst's active site and back, greatly influences, and in some cases determines, the catalyst's rate and overpotential, a measure of the energy efficiency of the reaction.
In the several classes of catalysts studied, the CME team showed that proton relays—which create paths for intra– and intermolecular proton transfers—regulate the movement of protons and electrons. The constitution, placement, and number of proton relays can accelerate reactions and dramatically improve their energy efficiency.
Tuning proton relays to the environment also influences the reaction. By "pKa matching," where the pKa, or proton-donating ability, of the solution is matched to that of the protonated pendant amine that serves as a proton relay, reactions can be sped up. For example, through pKa matching, the rate of hydrogen production by the Center's catalysts can increase by orders of magnitude. Matching the pKa is now recognized as a fundamental principle of catalyst design.
2. Controlling proton movement can lead to catalysts with rates that surpass enzymes. By designing a catalyst with an eye to where, when, why, and how protons move, CME researchers created a nickel-based catalyst that drives hydrogen production at rates exceeding 100,000 molecules per second.1 The catalyst is designed so that the protons are disfavored from landing at the "wrong" site, which would give less productive isomers. The catalyst produces H2 at rates exceeding that of hydrogenase enzymes, which produce hydrogen in algae and other microbes, though the energy efficiency (overpotential) of the natural enzyme is much better than that of the synthetic catalyst.
While the same rates have not been achieved with nitrogen reduction, placing proton relays into a tungsten-based catalyst with phosphorus and nitrogen ligands sped the reaction, doubling the amount of protonated ammonia (NH4+) produced.
3. …are reversible. Enzymes change hydrogen to protons and back on the fly. By designing in proton relays and other features, the CME team created a catalyst that is reversible and has a low overpotential. In designing this and other catalysts, a problem persisted—the need for a reliable procedure to accurately determine catalyst overpotentials and energy efficiencies. The researchers designed just such a procedure.2
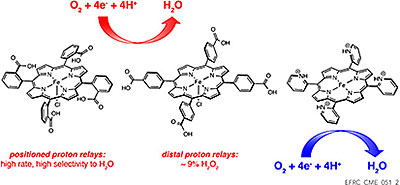
4. …and selective. In an iron-based catalyst for oxygen reduction, the catalyst exhibited much higher selectivity for the desired 4-electron reduction to H2O when carboxylic acid groups were positioned to interact with O2-derived ligands bound to iron, compared to a similar catalyst with distant relays.3 This selectivity results in higher energy efficiency and leads to much longer catalyst lifetime, as hydrogen peroxide that is often formed degrades the catalyst. Similar effects are observed for protonated pyridine relays. Further studies of these catalysts, especially state-of-the-art ab initio molecular dynamics simulations implicate a new mechanistic role for the relays: solvent organization to facilitate proton delivery.
5. Computational tools can predict catalyst performance and enable catalyst design. The CME scientists developed the capability to calculate the complete set of thermodynamic properties governing the chemistry of the team's nickel-based catalyst, abbreviated [Ni(PR2NR'2)2]2+ and nicknamed the DuBois catalyst, after former Deputy Center Director Daniel DuBois. They calculate thermochemical values deductively with an accuracy rarely obtained. The data and correlations among the values can be used to predict the relative free energies of all catalytic intermediates based on three easily determined quantities (two E° values and one pKa value). The derived "free-energy maps" and approach are general and powerful, allowing prediction and optimization of catalyst performance.
The researchers also made significant progress in developing an ab initio-derived microkinetic model of [Ni(PR2NR'2)2]2+, providing insight into the role of pendant amines as proton relays. The model includes all 29 elementary reactions that form the catalytic cycle4-9 and affords rationalization of aspects of the chemistry that enhance or diminish catalytic rates.
Where we've been, where we're going. Our results are the outcome of synergistic interactions among CME investigators in experiments and theory, yielding results only possible through the strong focus and intellectual cross-fertilization of ideas that an EFRC provides. The results have been published in 95 articles in peer-reviewed scientific journals, including the Journal of the American Chemical Society, Science, and Nature Chemistry. The first five years have gotten us this far. In the next four years, the team will work to make the hydrogen reactions faster and more efficient, to discover more selective catalysts to split molecular oxygen, and improve important aspects of molecular nitrogen catalysts.
References
- Helm, M. L.; Stewart, M. P.; Bullock, R. M.; Rakowski DuBois, M.; DuBois, D. L., Science 2011, 333, 863-866.
- Roberts, J. A. S.; Bullock, R. M., Inorg. Chem. 2013, 52, 3823-3835.
- Carver, C. T.; Matson, B. D.; Mayer, J. M., J. Am. Chem. Soc. 2012, 134, 5444-5447.
- Pool, D. H.; Stewart, M. P.; O'Hagan, M. J.; Shaw, W. J.; Roberts, J. A. S.; Bullock, R. M.; DuBois, D. L., Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 15634-15639.
- O'Hagan, M.; Shaw, W. J.; Raugei, S.; Chen, S.; Yang, J. Y.; Kilgore, U. J.; DuBois, D. L.; Bullock, R. M., J. Am. Chem. Soc. 2011, 133, 14301-14312.
- Raugei, S.; Chen, S.; Ho, M. H.; Ginovska-Pangovska, B.; Rousseau, R. J.; Dupuis, M.; DuBois, D. L.; Bullock, R. M., Chem. Eur. J. 2012, 18, 6493-6506.
- O'Hagan, M.; Ho, M. H.; Yang, J. Y.; Appel, A. M.; Rakowski DuBois, M.; Raugei, S.; Shaw, W. J.; DuBois, D. L.; Bullock, R. M., J. Am. Chem. Soc. 2012, 134, 19409-19424.
- Fernandez, L. E.; Horvath, S.; Hammes-Schiffer, S., J. Phys. Chem. C 2012, 116, 3171-3180.
- Ho, M.-H.; Chen, S.; Rousseau, R.; Dupuis, M.; Bullock, R. M.; Raugei, S., Bio-Inspired Molecular Catalysts for Hydrogen Oxidation and Hydrogen Production, in Applications of Molecular Modeling to Challenges in Clean Energy, Fitzgerald, G.; Govind, N., Eds. American Chemical Society: 2013; Vol. 1133, pp 89-111.

