In the Heat of the Reaction, a Single Atom Delivers
Converting carbon monoxide in fuel cells with a gold catalyst hinges on one ion shuttling the critical electron
(March 2015)
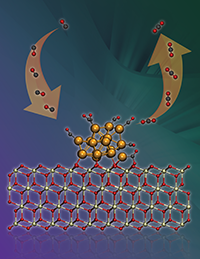
This research highlights the importance of the dynamic creation of active sites for converting carbon monoxide (left arrow) into carbon dioxide (right) under reaction conditions. Image credit: Cortland Johnson and Vanda Glezakou. Enlarge/expand Image.
Results: Not present when the reaction starts or ends, the driving force behind turning poisonous carbon monoxide into a benign form is a single atom that appears in the heat of action, according to scientists at Pacific Northwest National Laboratory. In the simulations, carbon monoxide binds with a gold nanoparticle on top of a cerium dioxide support. The carbon monoxide changes the surface; it spurs a single gold ion to leave the nanoparticle. The ion delivers an electron and returns to the particle.
"This is single-atom catalysis," said Dr. Roger Rousseau, a computational chemist at PNNL who co-led the study. "With a new twist -- the catalyst is dynamically formed under operating conditions."
Why It Matters: Clean air. Converting or oxidizing carbon monoxide is vital in fuel cells that create electricity without pollutants. It is also used in cleaning the air in firefighters' masks. Such oxidations could also apply to similar reactions used in manufacturing. Understanding exactly how the catalyst that drives this reaction works could open doors for improved efficiency and reduced costs.
"Unless you know what is happening, and when, and where, you really can't tune the properties of the catalysts to get what we need," said Dr. Vanda Glezakou, a computational chemist at PNNL who co-led the study.
Methods: For about two years, Rousseau, Glezakou, and their colleagues delved into understanding the mechanics of a metal catalyst and supporting material used to drive the oxidation of carbon monoxide. The reaction is completely tractable using computational simulations. Understanding the oxidation reaction will provide insights into fuel cells and air cleaning systems, as well as more complicated ketone and aldol reactions.
In the first year, the team built the protocol to model the cerium dioxide support, which performs electron transfer or reduction-oxidation reactions, along with a gold nanoparticle catalyst. "The protocol and the values we needed didn't exist in the literature," said Rousseau. "So, we had to start from scratch. These types of studies take more time and resources than typical calculation of energies."
With the energies and other parameters determined and the computational model built, the team ran numerous simulations. They used computers at EMSL, a U.S. Department of Energy scientific user facility.
The simulation ran ab initio molecular dynamics and static density functional theory calculations to investigate the reaction mechanism. What they found was quite unexpected -- a single-molecule dynamic catalyst.
The single gold cation, which only exists during the reaction, breaks off from the gold. It catalyzes the reaction and then returns to the gold. This unexpected phenomenon results from the single-atom site's ability to work with the redox properties of the support.
What's Next: The team is continuing to delve into the mechanism by which the metal particle and its support work. "What we are learning is that the supports work as hard as the catalyst," said Glezakou.
They are now working on catalysts and supports for adding hydrogen and removing oxygen to transform plant materials and other biomass into fuels.
Acknowledgments
Sponsors: This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences. JL and YGW were also financially supported by NKBRSF and NSFC of China.
User Facility:Computational resources were provided at EMSL, a national scientific user facility at PNNL and sponsored by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research and at the National Energy Research Scientific Computing Center (NERSC) at Lawrence Berkeley National Laboratory.
Research Area: Chemical Sciences
Research Team: Yang-Gang Wang, PNNL and Tsinghua University; Jun Li, Tsinghua University; Donghai Mei, Vassiliki-Alexandra Glezakou, and Roger Rousseau, PNNL.
Reference: Wang YG, D Mei, VA Glezakou, J Li, and R Rousseau. 2015. "Dynamic Formation of Single-atom Catalytic Active Sites during CO Oxidation on CeO2-supported Gold Nanoparticles." Nature Communications 6, article no. 6511. DOI: 10.1038/natcomms7511
