Finding the Perfect Spot to Rest
Scientists see carbon chains preferred locales on popular catalyst
(January 2010)
Results: Prima donnas. Floppy chains of carbon atoms are particular about where they want to be on a titanium dioxide catalyst, according to a new study from Pacific Northwest National Laboratory and the University of Texas at Austin. The catalyst's surface resembles corrugated cardboard: ridges of oxygen atoms run parallel to valleys of titanium atoms. Influenced by a weak attraction to the titanium, the hydrocarbon chain settles into the valleys.
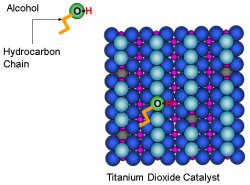
View Animation The scientists added alcohol molecules to the surface of the titanium dioxide catalyst. The alcohol's oxygen atom shed its hydrogen atom and slid into a vacant oxygen spot on the catalyst's surface.
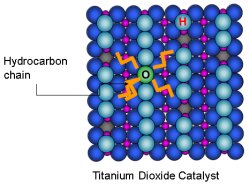
View Animation Influenced by a weak attraction to the titanium, the hydrocarbon chain settles into the valleys on the surface of the titanium dioxide.
Why it matters: The location of the hydrocarbon chain determines, in part, how well the model catalyst titanium dioxide will perform. Understanding where the carbon chain is and why is vital data for those seeking to modify catalyzed reactions; for example, making the reactions faster or more selective. "It has to be in the right place," said Dr. Roger Rousseau, a catalysis expert at Pacific Northwest National Laboratory. "It can't just be anywhere.
Methods: To determine where and why the hydrocarbon chain resides on the catalyst, the team conducted both experimental and theoretical studies. The project began with a scanning tunneling microscope (STM), which provided snapshots of the catalyst's surface. The microscope is located at the Department of Energy's EMSL, a national scientific user facility at PNNL.
The scientists added alcohol molecules to the surface. The alcohol's oxygen atom shed its hydrogen atom and slid into a vacant oxygen spot on the catalyst's surface. To understand how the length of the chain influenced its orientation, the researchers used alcohol molecules with eight carbon atoms and varied position of the hydroxyl (-OH) anchor from the very end to the middle of the hydrocarbon chain.
Using the STM, the scientists saw that the hydrocarbon chains, known as alkyl chains, had distinct preferences regarding where they resided.
Then, the theoretical chemists used the atomic-level information from the microscopy images to perform density functional theory calculations with a van der Waals correction. The calculations showed that the alkyl chains preference was based on a weak attraction between the carbon chains and the titanium atoms as well as a slight repulsion between the carbon and the oxygen atoms. These weak attractions are known as van der Waals forces.
Further, the scientists showed that the rotation of hydrocarbon chains anchored in the middle over the barrier of the oxygen rows is much harder than rotation of hydrocarbon chain anchored at the end. This is because when the anchor is in the middle, two hydrocarbon arms have to move over the oxygens as opposed to just one.
What's next: Knowing how titanium dioxide's surface influences the hydrocarbon chain's position, researchers are moving to investigate how the surface of another common catalyst, tungsten trioxide, reacts with alcohol molecules.
"This research is the first step to understanding how reactants align themselves for future reactions," said Dr. Bruce D. Kay, an expert in chemical reactions and molecular dynamics, who worked on the project.
Acknowledgments: This research was done by Dr. Roger Rousseau, Dr. Bruce D. Kay, and Dr. Zdenek Dohnalek of PNNL; Zhenrong Zhang, a postdoctoral fellow at Pacific Northwest National Laboratory who recently accepted a position at Baylor University; and Jinlong Gong at the University of Texas at Austin.
The research was funded by U.S. Department of Energy Office of Basic Energy Sciences.
Research was conducted in the Department of Energy's EMSL, a national scientific user facility at PNNL.
Reference: Zhang Z, R Rousseau, J Gong, BD Kay, and Z Dohnalek. 2009. "Imaging Hindered Rotations of Alkoxy Species on TiO2(110)." Journal of the American Chemical Society 131(49):17926-17932. DOI:10.1021/ja907431s.
